Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs
- PMID: 22829320
- PMCID: PMC3440212
- DOI: 10.1104/pp.112.202408
Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs
Erratum in
-
CORRECTION: Vol. 160: 379-395, 2012.Plant Physiol. 2018 Nov;178(3):1423. doi: 10.1104/pp.18.01168. Plant Physiol. 2018. PMID: 30425159 Free PMC article. No abstract available.
Abstract
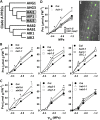
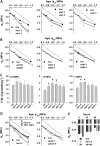
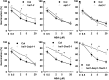
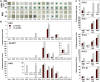
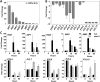
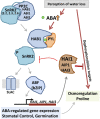
Six Arabidopsis (Arabidopsis thaliana) clade A protein phosphatase 2Cs (PP2Cs) have established abscisic acid (ABA) signaling roles; however, phenotypic roles of the remaining three "HAI" PP2Cs, Highly ABA-Induced1 (HAI1), AKT1-Interacting PP2C1/HAI2, and HAI3, have remained unclear. HAI PP2C mutants had enhanced proline and osmoregulatory solute accumulation at low water potential, while mutants of other clade A PP2Cs had no or lesser effect on these drought resistance traits. hai1-2 also had increased expression of abiotic stress-associated genes, including dehydrins and late embryogenesis abundant proteins, but decreased expression of several defense-related genes. Conversely, the HAI PP2Cs had relatively less impact on several ABA sensitivity phenotypes. HAI PP2C single mutants were unaffected in ABA sensitivity, while double and triple mutants were moderately hypersensitive in postgermination ABA response but ABA insensitive in germination. The HAI PP2Cs interacted most strongly with PYL5 and PYL7 to -10 of the PYL/RCAR ABA receptor family, with PYL7 to -10 interactions being relatively little affected by ABA in yeast two-hybrid assays. HAI1 had especially limited PYL interaction. Reduced expression of the main HAI1-interacting PYLs at low water potential when HAI1 expression was strongly induced also suggests limited PYL regulation and a role of HAI1 activity in negatively regulating specific drought resistance phenotypes. Overall, the HAI PP2Cs had greatest effect on ABA-independent low water potential phenotypes and lesser effect on classical ABA sensitivity phenotypes. Both this and their distinct PYL interaction demonstrate a new level of functional differentiation among the clade A PP2Cs and a point of cross talk between ABA-dependent and ABA-independent drought-associated signaling.
Figures



References
-
- Alexa A, Rahnenführer J, Lengauer T. (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607 - PubMed
-
- Bates LS, Waldren RP, Teare ID. (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207
-
- Blum A. (2005) Drought resistance, water-use efficiency and yield potential\x{2014}are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56: 1159–1168
-
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

