Application of micro-computed tomography with iodine staining to cardiac imaging, segmentation, and computational model development
- PMID: 22829390
- PMCID: PMC3493467
- DOI: 10.1109/TMI.2012.2209183
Application of micro-computed tomography with iodine staining to cardiac imaging, segmentation, and computational model development
Abstract
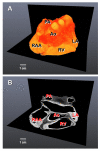
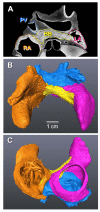
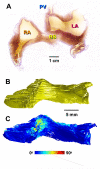
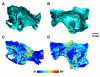
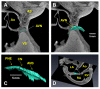
Micro-computed tomography (micro-CT) has been widely used to generate high-resolution 3-D tissue images from small animals nondestructively, especially for mineralized skeletal tissues. However, its application to the analysis of soft cardiovascular tissues has been limited by poor inter-tissue contrast. Recent ex vivo studies have shown that contrast between muscular and connective tissue in micro-CT images can be enhanced by staining with iodine. In the present study, we apply this novel technique for imaging of cardiovascular structures in canine hearts. We optimize the method to obtain high-resolution X-ray micro-CT images of the canine atria and its distinctive regions-including the Bachmann's bundle, atrioventricular node, pulmonary arteries and veins-with clear inter-tissue contrast. The imaging results are used to reconstruct and segment the detailed 3-D geometry of the atria. Structure tensor analysis shows that the arrangement of atrial fibers can also be characterized using the enhanced micro-CT images, as iodine preferentially accumulates within the muscular fibers rather than in connective tissues. This novel technique can be particularly useful in nondestructive imaging of 3-D cardiac architectures from large animals and humans, due to the combination of relatively high speed ( ~ 1 h/per scan of the large canine heart) and high voxel resolution (36 μm) provided. In summary, contrast micro-CT facilitates fast and nondestructive imaging and segmenting of detailed 3-D cardiovascular geometries, as well as measuring fiber orientation, which are crucial in constructing biophysically detailed computational cardiac models.
Figures


References
-
- Batchelor PG, Atkinson D, Hill DL, Calamante F, Connelly A. Anisotropic noise propagation in diffusion tensor MRI sampling schemes. Magn Reson Med. 2003;49:1143–51. - PubMed
-
- Benson AP, Aslanidi OV, Zhang H, Holden AV. The canine virtual ventricular wall: a platform for dissecting pharmacological effects on propagation and arrhythmogenesis. Prog Biophys Mol Biol. 2008;96:187–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

