Rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya
- PMID: 22829650
- PMCID: PMC3433858
- DOI: 10.1093/infdis/jis447
Rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya
Abstract
Background: To understand and model the impact of pneumococcal conjugate vaccines at the population level, we need to know the transmission dynamics of individual pneumococcal serotypes. We estimated serotype-specific clearance and acquisition rates of nasopharyngeal colonization among Kenyan children.
Methods: Children aged 3-59 months who were identified as carriers in a cross-sectional survey were followed-up approximately 1, 2, 4, 8, 16, and 32 days later and monthly thereafter until culture of 2 consecutive swabs yielded an alternative serotype or no pneumococcus. Serotype-specific clearance rates were estimated by exponential regression of interval-censored carriage durations. Duration was estimated as the reciprocal of the clearance rate, and acquisition rates were estimated on the basis of prevalence and duration, assuming an equilibrium state.
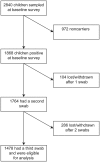
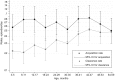
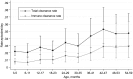
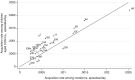
Results: Of 2840 children sampled between October 2006 and December 2008, 1868 were carriers. The clearance rate was 0.032 episodes/day (95% confidence interval [CI], .030-.034), for a carriage duration of 31.3 days, and the rate varied by serotype (P< .0005). Carriage durations for the 28 serotypes with ≥ 10 carriers ranged from 6.7 to 50 days. Clearance rates increased with year of age, adjusted for serotype (hazard ratio, 1.21; 95% CI, 1.15-1.27). The acquisition rate was 0.061 episodes/day (95% CI, .055-.067), which did not vary with age. Serotype-specific acquisition rates varied from 0.0002 to 0.0022 episodes/day. Serotype-specific acquisition rates correlated with prevalence (r=0.91; P< .00005) and with acquisition rates measured in a separate study involving 1404 newborns in Kilifi (r=0.87; P< .00005).
Conclusions: The large sample size and short swabbing intervals provide a precise description of the prevalence, duration, and acquisition of carriage of 28 pneumococcal serotypes. In Kilifi, young children experience approximately 8 episodes of carriage per year. The declining prevalence with age is attributable to increasing clearance rates.
Figures

References
-
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente vaccine study center group. Pediatr Infect Dis J. 2000;19:187–95. - PubMed
-
- Cutts FT, Zaman SMA, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. - PubMed
-
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. - PubMed
-
- O'Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–61. - PubMed
-
- Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;54:893–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

