Enterococcus faecalis produces abundant extracellular structures containing DNA in the absence of cell lysis during early biofilm formation
- PMID: 22829679
- PMCID: PMC3413405
- DOI: 10.1128/mBio.00193-12
Enterococcus faecalis produces abundant extracellular structures containing DNA in the absence of cell lysis during early biofilm formation
Abstract
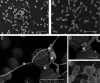
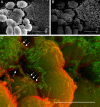
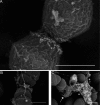
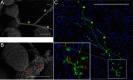
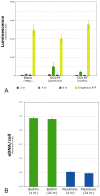
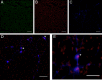
Enterococcus faecalis is a common Gram-positive commensal bacterium of the metazoan gastrointestinal tract capable of biofilm formation and an opportunistic pathogen of increasing clinical concern. Dogma has held that biofilms are slow-growing structures, often taking days to form mature microcolonies. Here we report that extracellular DNA (eDNA) is an integral structural component of early E. faecalis biofilms (≤4 h postinoculation). Combining cationic dye-based biofilm matrix stabilization techniques with correlative immuno-scanning electron microscopy (SEM) and fluorescent techniques, we demonstrate that--in early E. faecalis biofilms--eDNA localizes to previously undescribed intercellular filamentous structures, as well as to thick mats of extruded extracellular matrix material. Both of these results are consistent with previous reports that early biofilms are exquisitely sensitive to exogenous DNase treatment. High-resolution SEM demonstrates a punctate labeling pattern in both structures, suggesting the presence of an additional, non-DNA constituent. Notably, the previously described fratricidal or lytic mechanism reported as the source of eDNA in older (≥24 h) E. faecalis biofilms does not appear to be at work under these conditions; extensive visual examination by SEM revealed a striking lack of lysed cells, and bulk biochemical assays also support an absence of significant lysis at these early time points. In addition, some cells demonstrated eDNA labeling localized at the septum, suggesting the possibility of DNA secretion from metabolically active cells. Overall, these data are consistent with a model in which a subpopulation of viable E. faecalis cells secrete or extrude DNA into the extracellular matrix.
Importance: This paper reports the production of extracellular DNA during early biofilm formation in Enterococcus faecalis. The work is significant because the mechanism of eDNA (extracellular DNA) production is independent of cell lysis and the DNA is confined to well-defined structures, suggesting a novel form of DNA secretion by viable cells. Previous models of biofilm formation in enterococci and related species propose cell lysis as the mechanism of DNA release.
Figures
References
-
- Flemming HC, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8:623–633 - PubMed
-
- Lewis K. 2008. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 322:107–131 - PubMed
-
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

