Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway
- PMID: 22829774
- PMCID: PMC3400581
- DOI: 10.1371/journal.pgen.1002772
Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway
Abstract
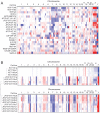
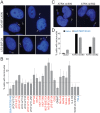
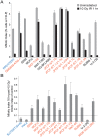
The Alternative Lengthening of Telomeres (ALT) pathway is a telomerase-independent pathway for telomere maintenance that is active in a significant subset of human cancers and in vitro immortalized cell lines. ALT is thought to involve templated extension of telomeres through homologous recombination, but the genetic or epigenetic changes that unleash ALT are not known. Recently, mutations in the ATRX/DAXX chromatin remodeling complex and histone H3.3 were found to correlate with features of ALT in pancreatic neuroendocrine cancers, pediatric glioblastomas, and other tumors of the central nervous system, suggesting that these mutations might contribute to the activation of the ALT pathway in these cancers. We have taken a comprehensive approach to deciphering ALT by applying genomic, molecular biological, and cell biological approaches to a panel of 22 ALT cell lines, including cell lines derived in vitro. Here we show that loss of ATRX protein and mutations in the ATRX gene are hallmarks of ALT-immortalized cell lines. In addition, ALT is associated with extensive genome rearrangements, marked micronucleation, defects in the G2/M checkpoint, and altered double-strand break (DSB) repair. These attributes will facilitate the diagnosis and treatment of ALT positive human cancers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274. - PubMed
-
- Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26:447–450. - PubMed
-
- Bechter OE, Shay JW, Wright WE. The Frequency of Homologous Recombination in Human ALT Cells. Cell Cycle. 2004;3:457–549. - PubMed
-
- Londono-Vallejo JA, Der-Sarkissian H, Cazes L, Bacchetti S, Reddel RR. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 2004;64:2324–2327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

