Oxidative Stress and the ER Stress Response in a Murine Model for Early-Stage Alcoholic Liver Disease
- PMID: 22829816
- PMCID: PMC3399426
- DOI: 10.1155/2012/207594
Oxidative Stress and the ER Stress Response in a Murine Model for Early-Stage Alcoholic Liver Disease
Abstract
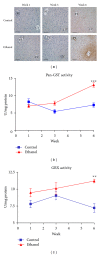
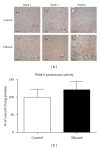
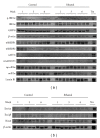
Alcoholic liver disease (ALD) is a primary cause of morbidity and mortality in the United States and constitutes a significant socioeconomic burden. Previous work has implicated oxidative stress and endoplasmic reticulum (ER) stress in the etiology of ALD; however, the complex and interrelated nature of these cellular responses presently confounds our understanding of ethanol-induced hepatopathy. In this paper, we assessed the pathological contribution of oxidative stress and ER stress in a time-course mouse model of early-stage ALD. Ethanol-treated mice exhibited significant hepatic panlobular steatosis and elevated plasma ALT values compared to isocaloric controls. Oxidative stress was observed in the ethanol-treated animals through a significant increase in hepatic TBARS and immunohistochemical staining of 4-HNE-modified proteins. Hepatic glutathione (GSH) levels were significantly decreased as a consequence of decreased CBS activity, increased GSH utilization, and increased protein glutathionylation. At the same time, immunoblot analysis of the PERK, IRE1α, ATF6, and SREBP pathways reveals no significant role for these UPR pathways in the etiology of hepatic steatosis associated with early-stage ALD. Collectively, our results indicate a primary pathogenic role for oxidative stress in the early initiating stages of ALD that precedes the involvement of the ER stress response.
Figures

References
-
- Oshea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. American Journal of Gastroenterology. 2010;105(1):14–32. - PubMed
-
- Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032–2039. - PubMed
-
- Arteel G, Marsano L, Mendez C, Bentley F, McClain CJ. Advances in alcoholic liver disease. Bailliere’s Best Practice and Research in Clinical Gastroenterology. 2003;17(4):625–647. - PubMed
-
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Archives of Toxicology. 2009;83(6):519–548. - PubMed
-
- De Minicis S, Brenner DA. Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. Journal of Gastroenterology and Hepatology. 2008;23(1, supplement):S98–S103. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

