Altered glucose homeostasis and hepatic function in obese mice deficient for both kinin receptor genes
- PMID: 22829877
- PMCID: PMC3400662
- DOI: 10.1371/journal.pone.0040573
Altered glucose homeostasis and hepatic function in obese mice deficient for both kinin receptor genes
Abstract
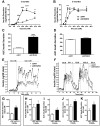
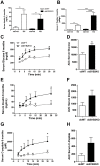
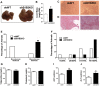
The Kallikrein-Kinin System (KKS) has been implicated in several aspects of metabolism, including the regulation of glucose homeostasis and adiposity. Kinins and des-Arg-kinins are the major effectors of this system and promote their effects by binding to two different receptors, the kinin B2 and B1 receptors, respectively. To understand the influence of the KKS on the pathophysiology of obesity and type 2 diabetes (T2DM), we generated an animal model deficient for both kinin receptor genes and leptin (obB1B2KO). Six-month-old obB1B2KO mice showed increased blood glucose levels. Isolated islets of the transgenic animals were more responsive to glucose stimulation releasing greater amounts of insulin, mainly in 3-month-old mice, which was corroborated by elevated serum C-peptide concentrations. Furthermore, they presented hepatomegaly, pronounced steatosis, and increased levels of circulating transaminases. This mouse also demonstrated exacerbated gluconeogenesis during the pyruvate challenge test. The hepatic abnormalities were accompanied by changes in the gene expression of factors linked to glucose and lipid metabolisms in the liver. Thus, we conclude that kinin receptors are important for modulation of insulin secretion and for the preservation of normal glucose levels and hepatic functions in obese mice, suggesting a protective role of the KKS regarding complications associated with obesity and T2DM.
Conflict of interest statement
Figures



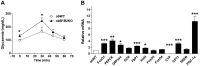
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

