Generalization of the dynamic clamp concept in neurophysiology and behavior
- PMID: 22829895
- PMCID: PMC3400657
- DOI: 10.1371/journal.pone.0040887
Generalization of the dynamic clamp concept in neurophysiology and behavior
Abstract
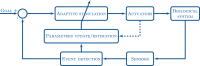
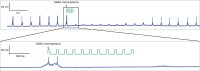
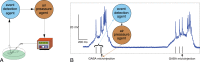
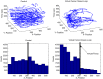
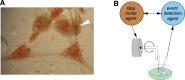
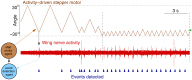
The idea of closed-loop interaction in in vitro and in vivo electrophysiology has been successfully implemented in the dynamic clamp concept strongly impacting the research of membrane and synaptic properties of neurons. In this paper we show that this concept can be easily generalized to build other kinds of closed-loop protocols beyond (or in addition to) electrical stimulation and recording in neurophysiology and behavioral studies for neuroethology. In particular, we illustrate three different examples of goal-driven real-time closed-loop interactions with drug microinjectors, mechanical devices and video event driven stimulation. Modern activity-dependent stimulation protocols can be used to reveal dynamics (otherwise hidden under traditional stimulation techniques), achieve control of natural and pathological states, induce learning, bridge between disparate levels of analysis and for a further automation of experiments. We argue that closed-loop interaction calls for novel real time analysis, prediction and control tools and a new perspective for designing stimulus-response experiments, which can have a large impact in neuroscience research.
Conflict of interest statement
Figures



References
-
- Marmont G. Studies on the axon membrane; a new method. J Cell Physiol. 1949;34:351–382. - PubMed
-
- Cole K. Electrochemistry in biology and medicine, Wiley, New York, chapter Ions, potentials and the nerve impulse. 1955. pp. 121–140.
-
- Robinson HP, Kawai N. Injection of digitally synthesized synaptic conductance transients to measure the integrative properties of neurons. J Neurosci Methods. 1993;49:157–165. - PubMed
-
- Sharp AA, O’Neil MB, Abbott LF, Marder E. The dynamic clamp: artificial conductances in biological neurons. Trends Neurosci. 1993;16:389–394. - PubMed
-
- Prinz AA, Abbott LF, Marder E. The dynamic clamp comes of age. Trends Neurosci. 2004;27:218–224. - PubMed

