Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice
- PMID: 22829897
- PMCID: PMC3400671
- DOI: 10.1371/journal.pone.0040914
Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice
Abstract
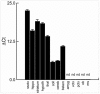
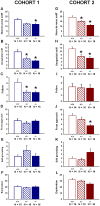
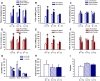
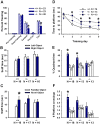
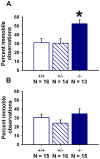
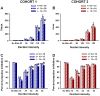
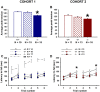
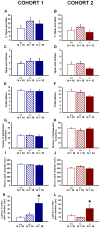
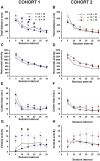
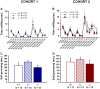
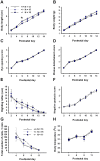
ENGRAILED 2 (En2), a homeobox transcription factor, functions as a patterning gene in the early development and connectivity of rodent hindbrain and cerebellum, and regulates neurogenesis and development of monoaminergic pathways. To further understand the neurobiological functions of En2, we conducted neuroanatomical expression profiling of En2 wildtype mice. RTQPCR assays demonstrated that En2 is expressed in adult brain structures including the somatosensory cortex, hippocampus, striatum, thalamus, hypothalamus and brainstem. Human genetic studies indicate that EN2 is associated with autism. To determine the consequences of En2 mutations on mouse behaviors, including outcomes potentially relevant to autism, we conducted comprehensive phenotyping of social, communication, repetitive, and cognitive behaviors. En2 null mutants exhibited robust deficits in reciprocal social interactions as juveniles and adults, and absence of sociability in adults, replicated in two independent cohorts. Fear conditioning and water maze learning were impaired in En2 null mutants. High immobility in the forced swim test, reduced prepulse inhibition, mild motor coordination impairments and reduced grip strength were detected in En2 null mutants. No genotype differences were found on measures of ultrasonic vocalizations in social contexts, and no stereotyped or repetitive behaviors were observed. Developmental milestones, general health, olfactory abilities, exploratory locomotor activity, anxiety-like behaviors and pain responses did not differ across genotypes, indicating that the behavioral abnormalities detected in En2 null mutants were not attributable to physical or procedural confounds. Our findings provide new insight into the role of En2 in complex behaviors and suggest that disturbances in En2 signaling may contribute to neuropsychiatric disorders marked by social and cognitive deficits, including autism spectrum disorders.
Conflict of interest statement
Figures
Similar articles
-
Chronic desipramine treatment rescues depression-related, social and cognitive deficits in Engrailed-2 knockout mice.Genes Brain Behav. 2014 Mar;13(3):286-298. doi: 10.1111/gbb.12115. Genes Brain Behav. 2014. PMID: 24730055 Free PMC article.
-
Aberrant Somatosensory Processing and Connectivity in Mice Lacking Engrailed-2.J Neurosci. 2019 Feb 20;39(8):1525-1538. doi: 10.1523/JNEUROSCI.0612-18.2018. Epub 2018 Dec 28. J Neurosci. 2019. PMID: 30593497 Free PMC article.
-
Loss of GABAergic neurons in the hippocampus and cerebral cortex of Engrailed-2 null mutant mice: implications for autism spectrum disorders.Exp Neurol. 2013 Sep;247:496-505. doi: 10.1016/j.expneurol.2013.01.021. Epub 2013 Jan 27. Exp Neurol. 2013. PMID: 23360806 Free PMC article.
-
Mouse behavioral assays relevant to the symptoms of autism.Brain Pathol. 2007 Oct;17(4):448-59. doi: 10.1111/j.1750-3639.2007.00096.x. Brain Pathol. 2007. PMID: 17919130 Free PMC article. Review.
-
Behavioral phenotypes of genetic mouse models of autism.Genes Brain Behav. 2016 Jan;15(1):7-26. doi: 10.1111/gbb.12256. Epub 2015 Oct 22. Genes Brain Behav. 2016. PMID: 26403076 Free PMC article. Review.
Cited by
-
Evaluation of a TrkB agonist on spatial and motor learning in the Ube3a mouse model of Angelman syndrome.Learn Mem. 2020 Aug 17;27(9):346-354. doi: 10.1101/lm.051201.119. Print 2020 Sep. Learn Mem. 2020. PMID: 32817301 Free PMC article.
-
Translational Mouse Models of Autism: Advancing Toward Pharmacological Therapeutics.Curr Top Behav Neurosci. 2016;28:1-52. doi: 10.1007/7854_2015_5003. Curr Top Behav Neurosci. 2016. PMID: 27305922 Free PMC article. Review.
-
Developmental Exposure to a Human-Relevant Polychlorinated Biphenyl Mixture Causes Behavioral Phenotypes That Vary by Sex and Genotype in Juvenile Mice Expressing Human Mutations That Modulate Neuronal Calcium.Front Neurosci. 2021 Dec 6;15:766826. doi: 10.3389/fnins.2021.766826. eCollection 2021. Front Neurosci. 2021. PMID: 34938155 Free PMC article.
-
Autism Pathogenesis: The Superior Colliculus.Front Neurosci. 2019 Jan 9;12:1029. doi: 10.3389/fnins.2018.01029. eCollection 2018. Front Neurosci. 2019. PMID: 30686990 Free PMC article.
-
Altered Behaviors and Impaired Synaptic Function in a Novel Rat Model With a Complete Shank3 Deletion.Front Cell Neurosci. 2019 Mar 26;13:111. doi: 10.3389/fncel.2019.00111. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30971895 Free PMC article.
References
-
- Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. - PubMed
-
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng C–X, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

