The effect of monthly sulfadoxine-pyrimethamine, alone or with azithromycin, on PCR-diagnosed malaria at delivery: a randomized controlled trial
- PMID: 22829919
- PMCID: PMC3400634
- DOI: 10.1371/journal.pone.0041123
The effect of monthly sulfadoxine-pyrimethamine, alone or with azithromycin, on PCR-diagnosed malaria at delivery: a randomized controlled trial
Abstract
Background: New regimens for intermittent preventive treatment in pregnancy (IPTp) against malaria are needed as the effectiveness of the standard two-dose sulfadoxine-pyrimethamine (SP) regimen is under threat. Previous trials have shown that IPTp with monthly SP benefits HIV-positive primi- and secundigravidae, but there is no conclusive evidence of the possible benefits of this regimen to HIV-negative women, or to a population comprising of both HIV-positive and -negative women of different gravidities.
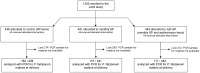
Methods: This study analyzed 484 samples collected at delivery as part of a randomized, partially placebo controlled clinical trial, conducted in rural Malawi between 2003 and 2007. The study included pregnant women regardless of their gravidity or HIV-infection status. The participants received SP twice (controls), monthly SP, or monthly SP and two doses of azithromycin (AZI-SP). The main outcome was the prevalence of peripheral Plasmodium falciparum malaria at delivery diagnosed with a real-time polymerase chain reaction (PCR) assay.
Findings: Overall prevalence of PCR-diagnosed peripheral P. falciparum malaria at delivery was 10.5%. Compared with the controls, participants in the monthly SP group had a risk ratio (95% CI) of 0.33 (0.17 to 0.64, P<0.001) and those in the AZI-SP group 0.23 (0.11 to 0.48, P<0.001) for malaria at delivery. When only HIV-negative participants were analyzed, the corresponding figures were 0.26 (0.12 to 0.57, P<0.001) for women in the monthly SP group, and 0.24 (0.11 to 0.53, P<0.001) for those in the AZI-SP group.
Conclusions: Our results suggest that increasing the frequency of SP administration during pregnancy improves the efficacy against malaria at delivery among HIV-negative women, as well as a population consisting of both HIV-positive and -negative pregnant women of all gravidities, in a setting of relatively low but holoendemic malaria transmission, frequent use of bed nets and high SP resistance.
Trial registration: ClinicalTrials.gov NCT00131235.
Conflict of interest statement
Figures
References
-
- Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. - PubMed
-
- Steketee RW, Nahlen BL, Parise ME, Menéndez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64 (suppl) 2001. 28 - PubMed
-
- World Health Organization (WHO) World Malaria Report 2011. Geneva: World Health Organization. 2011.
-
- Sridaran S, McClintock SK, Syphard LM, Herman KM, Barnwell J, et al. Anti-folate drug resistance in Africa: meta-analysis of reported dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant genotype frequencies in African Plasmodium falciparum parasite populations. Malar J. 2010;9:247. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical