Use of fluorochrome-labeled inhibitors of caspases to detect neuronal apoptosis in the whole-mounted lamprey brain after spinal cord injury
- PMID: 22829997
- PMCID: PMC3399409
- DOI: 10.1155/2012/835731
Use of fluorochrome-labeled inhibitors of caspases to detect neuronal apoptosis in the whole-mounted lamprey brain after spinal cord injury
Abstract
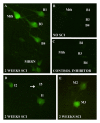
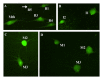
Apoptosis is a major feature in neural development and important in traumatic diseases. The presence of active caspases is a widely accepted marker of apoptosis. We report here the development of a method to study neuronal apoptotic death in whole-mounted brain preparations using fluorochrome-labeled inhibitors of caspases (FLICA). As a model we used axotomy-induced retrograde neuronal death in the CNS of larval sea lampreys. Once inside the cell, the FLICA reagents bind covalently to active caspases causing apoptotic cells to fluoresce, whereas nonapoptotic cells remain unstained. The fluorescent probe, the poly caspase inhibitor FAM-VAD-FMK, was applied to whole-mounted brain preparations of larval sea lampreys 2 weeks after a complete spinal cord (SC) transection. Specific labeling occurred only in identifiable spinal-projecting neurons of the brainstem previously shown to undergo apoptotic neuronal death at later times after SC transection. These neurons also exhibited intense labeling 2 weeks after a complete SC transection when a specific caspase-8 inhibitor (FAM-LETD-FMK) served as the probe. In this study we show that FLICA reagents can be used to detect specific activated caspases in identified neurons of the whole-mounted lamprey brain. Our results suggest that axotomy may cause neuronal apoptosis by activation of the extrinsic apoptotic pathway.
Figures


References
-
- Shifman MI, Jin L, Selzer ME. Regeneration in the lamprey spinal cord. In: Becker CG, Becker T, editors. Model Organisms in Spinal Cord Regeneration. Weinheim, Germany: Wiley-VCH Verlag GmbH; 2007. pp. 229–262.
-
- Cornide-Petronio ME, Ruiz MS, Barreiro-Iglesias A, Rodicio MC. Spontaneous regeneration of the serotonergic descending innervation in the sea lamprey after spinal cord injury. Journal of Neurotrauma. 2011;28:2535–2540. - PubMed
-
- Barreiro-Iglesias A. Targeting ependymal stem cells in vivo as a non-invasive therapy for spinal cord injury. DMM Disease Models and Mechanisms. 2010;3(11-12):667–668. - PubMed
-
- Barreiro-Iglesias A. ‘Evorego’: studying regeneration to understand evolution, the case of the serotonergic system. Brain, Behavior and Evolution. 2012;79:1–3. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

