Dynamic Association between HIV-1 Gag and Membrane Domains
- PMID: 22830021
- PMCID: PMC3399408
- DOI: 10.1155/2012/979765
Dynamic Association between HIV-1 Gag and Membrane Domains
Abstract
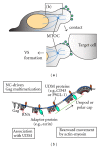
HIV-1 particle assembly is driven by the structural protein Gag. Gag binds to and multimerizes on the inner leaflet of the plasma membrane, eventually resulting in formation of spherical particles. During virus spread among T cells, Gag accumulates to the plasma membrane domain that, together with target cell membrane, forms a cell junction known as the virological synapse. While Gag association with plasma membrane microdomains has been implicated in virus assembly and cell-to-cell transmission, recent studies suggest that, rather than merely accumulating to pre-existing microdomains, Gag plays an active role in reorganizing the microdomains via its multimerization activity. In this paper, we will discuss this emerging view of Gag microdomain interactions. Relationships between Gag multimerization and microdomain association will be further discussed in the context of Gag localization to T-cell uropods and virological synapses.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
