Neural Circuit in the Dorsal Raphe Nucleus Responsible for Cannabinoid-Mediated Increases in 5-HT Efflux in the Nucleus Accumbens of the Rat Brain
- PMID: 22830043
- PMCID: PMC3399462
- DOI: 10.5402/2012/276902
Neural Circuit in the Dorsal Raphe Nucleus Responsible for Cannabinoid-Mediated Increases in 5-HT Efflux in the Nucleus Accumbens of the Rat Brain
Abstract
In vivo microdialysis was used in this study to reveal the role of cannabinoids in regulating serotonin (5-HT) efflux in the nucleus accumbens (NAcc) and dorsal raphe nucleus (DRN). The cannabinoid CB1 receptor agonists WIN55212-2 and CP55940 systematically administered to rats caused significant increases in 5-HT efflux in the NAcc but failed to have an effect in the DRN. To reveal mechanisms underlying regionally selective responses, we tested the hypothesis that cannabinoids have both direct and indirect effects on 5-HT efflux, depending on the location of CB1 receptors in the neural circuit between DRN and NAcc. We showed that the direct effect of cannabinoids caused a reduction in 5-HT efflux whereas the indirect effect resulted in an increase. Furthermore, the indirect effect was blocked by the GABA(A) receptor antagonist bicuculline in the DRN, suggesting that the action is likely due to a presynaptic inhibition on GABAergic activity that exerts a tonic influence on neuronal circuits regulating 5-HT efflux. Involvement of GABAergic neurons was confirmed by measuring changes in GABA efflux. Taken together, our study suggests that cannabinoids may have direct and indirect effects on the 5-HT regulatory circuits, resulting in regionally selective changes of 5-HT efflux in the brain.
Figures
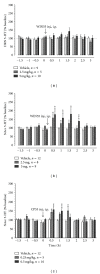
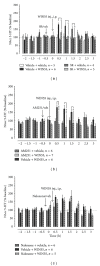

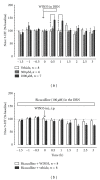

References
-
- Soares VDP, Campos AC, Bortoli VCD, Zangrossi H, Guimarães FS, Zuardi AW. Intra-dorsal periaqueductal gray administration of cannabidiol blocks panic-like response by activating 5-HT1A receptors. Behavioural Brain Research. 2010;213(2):225–229. - PubMed
-
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends in Pharmacological Sciences. 2001;22(11):565–572. - PubMed
-
- Pistis M, Muntoni AL, Pillolla G, Gessa GL. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. European Journal of Neuroscience. 2002;15(11):1795–1802. - PubMed
-
- Wallmichrath I, Szabo B. Analysis of the effect of cannabinoids on GABAergic neurotransmission in the substantia nigra pars reticulata. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2002;365(4):326–334. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
