Biology and clinical management of myeloproliferative neoplasms and development of the JAK inhibitor ruxolitinib
- PMID: 22830345
- PMCID: PMC3480698
- DOI: 10.2174/092986712803251511
Biology and clinical management of myeloproliferative neoplasms and development of the JAK inhibitor ruxolitinib
Abstract
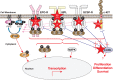
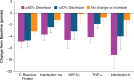
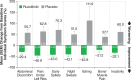
Myeloproliferative neoplasms (MPN) are debilitating stem cell-derived clonal myeloid malignancies. Conventional treatments for the BCR-ABL1-negative MPN including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) have, so far, been unsatisfactory. Following the discovery of dysregulated JAK-STAT signaling in patients with MPN, many efforts have been directed toward the development of molecularly targeted therapies, including inhibitors of JAK1 and JAK2. Ruxolitinib (previously known as INCB018424; Incyte Corporation, Wilmington, Delaware, USA) is a rationally designed potent oral JAK1 and JAK2 inhibitor that has undergone clinical trials in patients with PV, ET, and PMF. Ruxolitinib was approved on November 16, 2011 by the United States Food and Drug Administration for the treatment of intermediate or high-risk myelofibrosis (MF), including patients with PMF, post-PV MF, and post-ET MF. In randomized phase III studies, ruxolitinib treatment resulted in significant and durable reductions in splenomegaly and improvements in disease-related symptoms in patients with MF compared with placebo or best available therapy. The most common adverse events were anemia and thrombocytopenia, which were manageable and rarely led to discontinuation. This review addresses the cellular and molecular biology, and the clinical management of MPN.
Figures



References
-
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1 ):14–22. - PubMed
-
- Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, Barosi G, Verstovsek S, Birgegard G, Mesa R, Reilly JT, Gisslinger H, Vannucchi AM, Cervantes F, Finazzi G, Hoffman R, Gilliland DG, Bloomfield CD, Vardiman JW. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110(4 ):1092–1097. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464 ):1054–1061. - PubMed
-
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037 ):1144–1148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
