Glutamine supplementation in a child with inherited GS deficiency improves the clinical status and partially corrects the peripheral and central amino acid imbalance
- PMID: 22830360
- PMCID: PMC3495849
- DOI: 10.1186/1750-1172-7-48
Glutamine supplementation in a child with inherited GS deficiency improves the clinical status and partially corrects the peripheral and central amino acid imbalance
Abstract
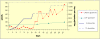
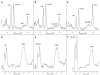
Glutamine synthetase (GS) is ubiquitously expressed in mammalian organisms and is a key enzyme in nitrogen metabolism. It is the only known enzyme capable of synthesising glutamine, an amino acid with many critical roles in the human organism. A defect in GLUL, encoding for GS, leads to congenital systemic glutamine deficiency and has been described in three patients with epileptic encephalopathy. There is no established treatment for this condition.Here, we describe a therapeutic trial consisting of enteral and parenteral glutamine supplementation in a four year old patient with GS deficiency. The patient received increasing doses of glutamine up to 1020 mg/kg/day. The effect of this glutamine supplementation was monitored clinically, biochemically, and by studies of the electroencephalogram (EEG) as well as by brain magnetic resonance imaging and spectroscopy.Treatment was well tolerated and clinical monitoring showed improved alertness. Concentrations of plasma glutamine normalized while levels in cerebrospinal fluid increased but remained below the lower reference range. The EEG showed clear improvement and spectroscopy revealed increasing concentrations of glutamine and glutamate in brain tissue. Concomitantly, there was no worsening of pre-existing chronic hyperammonemia.In conclusion, supplementation of glutamine is a safe therapeutic option for inherited GS deficiency since it corrects the peripheral biochemical phenotype and partially also improves the central biochemical phenotype. There was some clinical improvement but the patient had a long standing severe encephalopathy. Earlier supplementation with glutamine might have prevented some of the neuronal damage.
Figures


Similar articles
-
Minireview on Glutamine Synthetase Deficiency, an Ultra-Rare Inborn Error of Amino Acid Biosynthesis.Biology (Basel). 2016 Oct 19;5(4):40. doi: 10.3390/biology5040040. Biology (Basel). 2016. PMID: 27775558 Free PMC article. Review.
-
Secondary NAD+ deficiency in the inherited defect of glutamine synthetase.J Inherit Metab Dis. 2015 Nov;38(6):1075-83. doi: 10.1007/s10545-015-9846-4. Epub 2015 Apr 21. J Inherit Metab Dis. 2015. PMID: 25896882
-
Inborn error of amino acid synthesis: human glutamine synthetase deficiency.J Inherit Metab Dis. 2006 Apr-Jun;29(2-3):352-8. doi: 10.1007/s10545-006-0256-5. J Inherit Metab Dis. 2006. PMID: 16763901 Review.
-
Homozygous GLUL deletion is embryonically viable and leads to glutamine synthetase deficiency.Clin Genet. 2020 Dec;98(6):613-619. doi: 10.1111/cge.13844. Epub 2020 Oct 1. Clin Genet. 2020. PMID: 32888207
-
A Very Rare Etiology of Hypotonia and Seizures: Congenital Glutamine Synthetase Deficiency.Neuropediatrics. 2019 Feb;50(1):51-53. doi: 10.1055/s-0038-1675637. Epub 2018 Nov 15. Neuropediatrics. 2019. PMID: 30440076
Cited by
-
Carbohydrate and Glutamine Supplementation Attenuates the Increase in Rating of Perceived Exertion during Intense Exercise in Hypoxia Similar to 4200 m.Nutrients. 2020 Dec 11;12(12):3797. doi: 10.3390/nu12123797. Nutrients. 2020. PMID: 33322280 Free PMC article. Clinical Trial.
-
Minireview on Glutamine Synthetase Deficiency, an Ultra-Rare Inborn Error of Amino Acid Biosynthesis.Biology (Basel). 2016 Oct 19;5(4):40. doi: 10.3390/biology5040040. Biology (Basel). 2016. PMID: 27775558 Free PMC article. Review.
-
The Amino Acid Transporters of the Glutamate/GABA-Glutamine Cycle and Their Impact on Insulin and Glucagon Secretion.Front Endocrinol (Lausanne). 2013 Dec 31;4:199. doi: 10.3389/fendo.2013.00199. Front Endocrinol (Lausanne). 2013. PMID: 24427154 Free PMC article. Review.
-
Asparagine synthetase deficiency detected by whole exome sequencing causes congenital microcephaly, epileptic encephalopathy and psychomotor delay.Metab Brain Dis. 2015 Jun;30(3):687-94. doi: 10.1007/s11011-014-9618-0. Epub 2014 Sep 17. Metab Brain Dis. 2015. PMID: 25227173 Free PMC article.
-
Slc38a1 Conveys Astroglia-Derived Glutamine into GABAergic Interneurons for Neurotransmitter GABA Synthesis.Cells. 2020 Jul 13;9(7):1686. doi: 10.3390/cells9071686. Cells. 2020. PMID: 32668809 Free PMC article.
References
-
- Häussinger D. Hepatic glutamine transport and metabolism. Adv Enzymol Relat Areas Mol Biol. 1998;72:43–86. - PubMed
-
- Watford M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr. 2008;138:2003S–2007S. - PubMed
-
- Labow BI, Souba WW, Abcouwer SF. Mechanisms governing the expression of the enzymes of glutamine metabolism--glutaminase and glutamine synthetase. J Nutr. 2001;131:2467S–2474S. discussion 2486S-2467S. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

