Mutations in the gene PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions
- PMID: 22832103
- PMCID: PMC3334308
- DOI: 10.1016/j.celrep.2011.11.001
Mutations in the gene PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions
Abstract
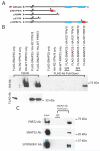
Paroxysmal kinesigenic dyskinesia with infantile convulsions (PKD/IC) is an episodic movement disorder with autosomal-dominant inheritance and high penetrance, but the causative genetic mutation is unknown. We have now identified four truncating mutations involving the gene PRRT2 in the vast majority (24/25) of well-characterized families with PKD/IC. PRRT2 truncating mutations were also detected in 28 of 78 additional families. PRRT2 encodes a proline-rich transmembrane protein of unknown function that has been reported to interact with the t-SNARE, SNAP25. PRRT2 localizes to axons but not to dendritic processes in primary neuronal culture, and mutants associated with PKD/IC lead to dramatically reduced PRRT2 levels, leading ultimately to neuronal hyperexcitability that manifests in vivo as PKD/IC.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Abe T, Kobayashi M, Araki K, Kodama H, Fujita Y, Shinozaki T, Ushijima H. Infantile convulsions with mild gastroenteritis. Brain & development. 2000;22:301–306. - PubMed
-
- Bennett LB, Roach ES, Bowcock AM. A locus for paroxysmal kinesigenic dyskinesia maps to human chromosome 16. Neurology. 2000;54:125–130. - PubMed
-
- Bhatia KP. Paroxysmal dyskinesias. Mov Disord. 2011;26:1157–1165. - PubMed
-
- Blakeley J, Jankovic J. Secondary paroxysmal dyskinesias. Mov Disord. 2002;17:726–734. - PubMed
-
- Bruno MK, Hallett M, Gwinn-Hardy K, Sorensen B, Considine E, Tucker S, Lynch DR, Mathews KD, Swoboda KJ, Harris J, et al. Clinical evaluation of idiopathic paroxysmal kinesigenic dyskinesia: new diagnostic criteria. Neurology. 2004;63:2280–2287. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

