Design and synthesis of Pictet-Spengler condensation products that exhibit oncogenic-RAS synthetic lethality and induce non-apoptotic cell death
- PMID: 22832321
- PMCID: PMC3423582
- DOI: 10.1016/j.bmcl.2012.06.077
Design and synthesis of Pictet-Spengler condensation products that exhibit oncogenic-RAS synthetic lethality and induce non-apoptotic cell death
Abstract
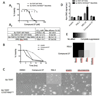
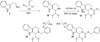
A series of Pictet-Spengler condensation derivatives (tetrahydro-β-carbolines) was designed, synthesized and evaluated for lethality against a panel of seven cancer cell lines. Seven compounds (2a, 13, 20, 21, 27, 29 and 34) showed lethality in at least five cell lines. Among these, compound 27 showed a unique selectivity towards oncogenic-RAS expressing BJ-TERT/LT/ST/RAS(V12) tumor cells, compared to non-transformed BJ-TERT cells. Further investigation revealed that 27 induces cell death without activation of caspases. This represents a useful new probe of non-apoptotic cell death and oncogenic-RAS synthetic lethality.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
New 3-tetrazolyl-β-carbolines and β-carboline-3-carboxylates with anti-cancer activity.Eur J Med Chem. 2019 Oct 1;179:123-132. doi: 10.1016/j.ejmech.2019.05.085. Epub 2019 May 31. Eur J Med Chem. 2019. PMID: 31247374
-
[Synthesis of 1-indole substituted beta-carboline alkaloid and its derivatives and evaluation of their preliminary antitumor activities].Yao Xue Xue Bao. 2004 Apr;39(4):259-62. Yao Xue Xue Bao. 2004. PMID: 15303653 Chinese.
-
[Synthesis and antitumor activities of 1-(4-chlorophenyl)-beta-carboline derivatives].Yao Xue Xue Bao. 2013 Jan;48(1):77-82. Yao Xue Xue Bao. 2013. PMID: 23600145 Chinese.
-
Recent insights into synthetic β-carbolines with anti-cancer activities.Eur J Med Chem. 2017 Dec 15;142:48-73. doi: 10.1016/j.ejmech.2017.05.059. Epub 2017 May 30. Eur J Med Chem. 2017. PMID: 28583770 Review.
-
A review of synthetic bioactive tetrahydro-β-carbolines: A medicinal chemistry perspective.Eur J Med Chem. 2021 Dec 5;225:113815. doi: 10.1016/j.ejmech.2021.113815. Epub 2021 Aug 30. Eur J Med Chem. 2021. PMID: 34479038 Review.
Cited by
-
Synthetic pathways to create asymmetric center at C1 position of 1-substituted-tetrahydro-β-carbolines - a review.RSC Adv. 2024 Sep 19;14(41):29827-29847. doi: 10.1039/d4ra05961a. eCollection 2024 Sep 18. RSC Adv. 2024. PMID: 39301229 Free PMC article. Review.
-
Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death.Oncotarget. 2015 Sep 15;6(27):24393-403. doi: 10.18632/oncotarget.5162. Oncotarget. 2015. PMID: 26405158 Free PMC article.
-
Convenient construction of tetrahydrochromeno[4',3':2,3]indolizino[8,7-b]indoles and tetrahydroindolizino[8,7-b]indoles via one-pot domino reaction.RSC Adv. 2018 Aug 14;8(50):28736-28744. doi: 10.1039/c8ra05138k. eCollection 2018 Aug 7. RSC Adv. 2018. PMID: 35542494 Free PMC article.
-
Role of Indole Scaffolds as Pharmacophores in the Development of Anti-Lung Cancer Agents.Molecules. 2020 Apr 1;25(7):1615. doi: 10.3390/molecules25071615. Molecules. 2020. PMID: 32244744 Free PMC article. Review.
-
Pharmacological importance of optically active tetrahydro-β-carbolines and synthetic approaches to create the C1 stereocenter.Molecules. 2014 Jan 27;19(2):1544-67. doi: 10.3390/molecules19021544. Molecules. 2014. PMID: 24473212 Free PMC article. Review.
References
-
-
There are numerous reports. For examples, see: Song Y, Wang J, Teng SF, Kesuma D, Deng Y, Duan J, Wang JH, Qi RZ, Sim MM. Bioorg. Med. Chem. Lett. 2002;12:1129. Liangxian C, Samit H, Langdon M, Thomas D. WO 2008127715. PTC Therapeutics, Inc.; USA: Carboline derivatives useful in the treatment of cancer and other diseases, PCT Int. Appl. 2008 Brent S, Seok WY. WO 2008103470. USA: Trustees of Columbia University in the City of New York; Preparation of carboline carboxylate derivatives as antitumor agents and oncogenic-RAS-signal dependent lethal compounds. PCT Int. Appl. 2008
-
-
- Ishida J, Wang HK, Bastow KF, Hu CQ, Lee KH. Bioorg. Med. Chem. Lett. 1999;9:3319. - PubMed
-
- Sakai R, Higa T, Jefford CW, Bernardinelli G. J. Am. Chem. Soc. 1986;108:6404.
-
- Rinehart KL, Kobayashi J, Harbour GC, Hughes RG, Jr, Mizsak SA, Scahill TA. J. Am. Chem. Soc. 1984;106:1524.
- Kobayashi J, Harbour GC, Gilmore J, Rinehart KL., Jr J. Am. Chem. Soc. 1984;106:1526.
-
- Leteurtre F, Madalengoitia JS, Orr A, Guzi TJ, Lehnert EK, Macdonald TL, Pommier Y. Cancer Res. 1992;52:4478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

