A peptide derived from the highly conserved protein GAPDH is involved in tissue protection by different antifungal strategies and epithelial immunomodulation
- PMID: 22832495
- PMCID: PMC3488162
- DOI: 10.1038/jid.2012.254
A peptide derived from the highly conserved protein GAPDH is involved in tissue protection by different antifungal strategies and epithelial immunomodulation
Abstract
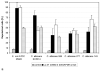
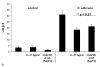
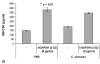
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has an important role not only in glycolysis but also in nonmetabolic processes, including transcription activation and apoptosis. We report the isolation of a human GAPDH (hGAPDH) (2-32) fragment peptide from human placental tissue exhibiting antimicrobial activity. The peptide was internalized by cells of the pathogenic yeast Candida albicans and initiated a rapid apoptotic mechanism, leading to killing of the fungus. Killing was dose-dependent, with 10 μg ml (3.1 μM) and 100 μg ml hGAPDH (2-32) depolarizing 45% and 90% of the fungal cells in a population, respectively. Experimental C. albicans infection induced epithelial hGAPDH (2-32) expression. Addition of the peptide significantly reduced the tissue damage as compared with untreated experimental infection. Secreted aspartic proteinase (Sap) activity of C. albicans was inhibited by the fragment at higher concentrations, with a median effective dose of 160 mg l(-1) (50 μM) for Sap1p and 200 mg l(-1) (63 μM) for Sap2p, whereas Sap3 was not inhibited at all. Interestingly, hGAPDH (2-32) induced significant epithelial IL-8 and GM-CSF secretion and stimulated Toll-like receptor 4 expression at low concentrations independently of the presence of C. albicans, without any toxic mucosal effects. In the future, the combination of different antifungal strategies, e.g., a conventional fungicidal with immunomodulatory effects and the inhibition of fungal virulence factors, might be a promising treatment option.
Figures
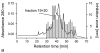

















Similar articles
-
Engineering improved variants of the antifungal peptide histatin 5 with reduced susceptibility to Candida albicans secreted aspartic proteases and enhanced antimicrobial potency.FEBS J. 2018 Jan;285(1):146-159. doi: 10.1111/febs.14327. Epub 2017 Nov 29. FEBS J. 2018. PMID: 29143452 Free PMC article.
-
Aspartic protease inhibitors as potential anti-Candida albicans drugs: impacts on fungal biology, virulence and pathogenesis.Curr Med Chem. 2011;18(16):2401-19. doi: 10.2174/092986711795843182. Curr Med Chem. 2011. PMID: 21568917 Review.
-
Granulocyte-macrophage colony-stimulating factor responses of oral epithelial cells to Candida albicans.Oral Microbiol Immunol. 2003 Jun;18(3):165-70. doi: 10.1034/j.1399-302x.2003.00061.x. Oral Microbiol Immunol. 2003. PMID: 12753468
-
Candida albicans VPS4 contributes differentially to epithelial and mucosal pathogenesis.Virulence. 2014;5(8):810-8. doi: 10.4161/21505594.2014.956648. Epub 2014 Oct 31. Virulence. 2014. PMID: 25483774 Free PMC article.
-
Targeting adhesion in fungal pathogen Candida albicans.Future Med Chem. 2021 Feb;13(3):313-334. doi: 10.4155/fmc-2020-0052. Epub 2020 Jun 22. Future Med Chem. 2021. PMID: 32564615 Review.
Cited by
-
Beyond Conventional Antifungals: Combating Resistance Through Novel Therapeutic Pathways.Pharmaceuticals (Basel). 2025 Mar 4;18(3):364. doi: 10.3390/ph18030364. Pharmaceuticals (Basel). 2025. PMID: 40143141 Free PMC article. Review.
-
Determination of the Relationships between the Chemical Structure and Antimicrobial Activity of a GAPDH-Related Fish Antimicrobial Peptide and Analogs Thereof.Antibiotics (Basel). 2022 Feb 23;11(3):297. doi: 10.3390/antibiotics11030297. Antibiotics (Basel). 2022. PMID: 35326761 Free PMC article.
-
Human antimicrobial peptides and proteins.Pharmaceuticals (Basel). 2014 May 13;7(5):545-94. doi: 10.3390/ph7050545. Pharmaceuticals (Basel). 2014. PMID: 24828484 Free PMC article.
-
Diversity and Molecular Evolution of Antimicrobial Peptides in Caecilian Amphibians.Toxins (Basel). 2024 Mar 14;16(3):150. doi: 10.3390/toxins16030150. Toxins (Basel). 2024. PMID: 38535816 Free PMC article.
-
Innate Defense against Fungal Pathogens.Cold Spring Harb Perspect Med. 2014 Nov 10;5(6):a019620. doi: 10.1101/cshperspect.a019620. Cold Spring Harb Perspect Med. 2014. PMID: 25384766 Free PMC article. Review.
References
-
- Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–95. - PubMed
-
- Borelli C, Ruge E, Lee JH, Schaller M, Vogelsang A, Monod M, et al. X-ray structures of Sap1 and Sap5: structural comparison of the secreted aspartic proteinases from Candida albicans. Proteins. 2008;72:1308–19. - PubMed
-
- Braga-Silva LA, Santos AL. Aspartic protease inhibitors as potential anti-Candida albicans drugs: impacts on fungal biology, virulence and pathogenesis. Curr Med Chem. 2011;18:2401–19. - PubMed
-
- Dalle F, Wachtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–71. - PubMed
-
- Dumke R, Hausner M, Jacobs E. Role of Mycoplasma pneumoniae glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in mediating interactions with the human extracellular matrix. Microbiology. 2011;157:2328–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

