Intravenous injection of neural progenitor cells improved depression-like behavior after cerebral ischemia
- PMID: 22832603
- PMCID: PMC3309503
- DOI: 10.1038/tp.2011.32
Intravenous injection of neural progenitor cells improved depression-like behavior after cerebral ischemia
Abstract
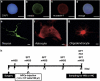
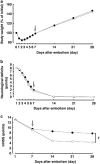
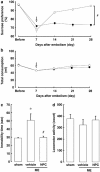
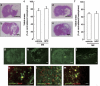
Poststroke depression (PSD) occurs in approximately one-third of stroke survivors and is one of the serious sequelae of stroke. The onset of PSD causes delayed functional recovery by rehabilitation and also increases cognitive impairment. However, appropriate strategies for the therapy against ischemia-induced depression-like behaviors still remain to be developed. Such behaviors have been associated with a reduced level of brain-derived neurotrophic factor (BDNF). In addition, accumulating evidence indicates the ability of stem cells to improve cerebral ischemia-induced brain injuries. However, it remains to be clarified as to the effect of neural progenitor cells (NPCs) on PSD and the association between BDNF level and PSD. Using NPCs, we investigated the effect of intravenous injection of NPCs on PSD. We showed that injection of NPCs improved ischemia-induced depression-like behaviors in the forced-swimming test and sucrose preference test without having any effect on the viable area between vehicle- and NPC-injected ischemic rats. The injection of NPCs prevented the decrease in the level of BDNF in the ipsilateral hemisphere. The levels of phosphorylated CREB, ERK and Akt, which have been implicated in events downstream of BDNF signaling, were also decreased after cerebral ischemia. NPC injection inhibited these decreases in the phosphorylation of CREB and ERK, but not that of Akt. Our findings provide evidence that injection of NPCs may have therapeutic potential for the improvement of depression-like behaviors after cerebral ischemia and that these effects might be associated with restoring BDNF-ERK-CREB signaling.
Figures
Similar articles
-
Intravenous injection of neural progenitor cells improves cerebral ischemia-induced learning dysfunction.Biol Pharm Bull. 2011;34(2):260-5. doi: 10.1248/bpb.34.260. Biol Pharm Bull. 2011. PMID: 21415538
-
Saikosaponin A improved depression-like behavior and inhibited hippocampal neuronal apoptosis after cerebral ischemia through p-CREB/BDNF pathway.Behav Brain Res. 2021 Apr 9;403:113138. doi: 10.1016/j.bbr.2021.113138. Epub 2021 Jan 22. Behav Brain Res. 2021. PMID: 33493495
-
Injection of neural progenitor cells attenuates decrease in level of connexin 43 in brain capillaries after cerebral ischemia.Neurosci Lett. 2013 May 24;543:152-6. doi: 10.1016/j.neulet.2013.03.053. Epub 2013 Apr 9. Neurosci Lett. 2013. PMID: 23583594
-
Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments.Neuro Endocrinol Lett. 2014;35(2):104-9. Neuro Endocrinol Lett. 2014. PMID: 24878979 Review.
-
Decreased Serum Brain-Derived Neurotrophic Factor May Indicate the Development of Poststroke Depression in Patients with Acute Ischemic Stroke: A Meta-Analysis.J Stroke Cerebrovasc Dis. 2018 Mar;27(3):709-715. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.003. Epub 2017 Nov 8. J Stroke Cerebrovasc Dis. 2018. PMID: 29128330 Review.
Cited by
-
Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic?Biomed Res Int. 2015;2015:519830. doi: 10.1155/2015/519830. Epub 2015 Feb 19. Biomed Res Int. 2015. PMID: 25789320 Free PMC article. Review.
-
Shuyu capsules relieve liver-qi depression by regulating ERK-CREB-BDNF signal pathway in central nervous system of rat.Exp Ther Med. 2017 Nov;14(5):4831-4838. doi: 10.3892/etm.2017.5125. Epub 2017 Sep 18. Exp Ther Med. 2017. PMID: 29201187 Free PMC article.
-
Panax notoginseng Saponins Stimulates Neurogenesis and Neurological Restoration After Microsphere-Induced Cerebral Embolism in Rats Partially Via mTOR Signaling.Front Pharmacol. 2022 Jun 13;13:889404. doi: 10.3389/fphar.2022.889404. eCollection 2022. Front Pharmacol. 2022. PMID: 35770087 Free PMC article.
-
Possible Involvement of PI3-K/Akt-Dependent GSK-3β Signaling in Proliferation of Neural Progenitor Cells After Hypoxic Exposure.Mol Neurobiol. 2019 Mar;56(3):1946-1956. doi: 10.1007/s12035-018-1216-4. Epub 2018 Jul 6. Mol Neurobiol. 2019. PMID: 29981053
-
Involvement of GSK-3β Phosphorylation Through PI3-K/Akt in Cerebral Ischemia-Induced Neurogenesis in Rats.Mol Neurobiol. 2017 Dec;54(10):7917-7927. doi: 10.1007/s12035-016-0290-8. Epub 2016 Nov 19. Mol Neurobiol. 2017. PMID: 27866373 Free PMC article.
References
-
- Stroke—1989 Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. - PubMed
-
- Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. - PubMed
-
- Burvill PW, Johnson GA, Jamrozik KD, Anderson CS, Stewart-Wynne EG, Chakera TM. Prevalence of depression after stroke: the Perth Community Stroke Study. Br J Psychiatry. 1995;166:320–327. - PubMed
-
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. - PubMed
-
- Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

