Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia
- PMID: 22832859
- PMCID: PMC3316151
- DOI: 10.1038/tp.2012.15
Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia
Abstract
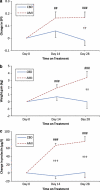
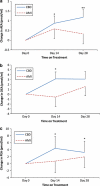
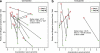
Cannabidiol is a component of marijuana that does not activate cannabinoid receptors, but moderately inhibits the degradation of the endocannabinoid anandamide. We previously reported that an elevation of anandamide levels in cerebrospinal fluid inversely correlated to psychotic symptoms. Furthermore, enhanced anandamide signaling let to a lower transition rate from initial prodromal states into frank psychosis as well as postponed transition. In our translational approach, we performed a double-blind, randomized clinical trial of cannabidiol vs amisulpride, a potent antipsychotic, in acute schizophrenia to evaluate the clinical relevance of our initial findings. Either treatment was safe and led to significant clinical improvement, but cannabidiol displayed a markedly superior side-effect profile. Moreover, cannabidiol treatment was accompanied by a significant increase in serum anandamide levels, which was significantly associated with clinical improvement. The results suggest that inhibition of anandamide deactivation may contribute to the antipsychotic effects of cannabidiol potentially representing a completely new mechanism in the treatment of schizophrenia.
Figures

References
-
- Rogers DP, Goldsmith CA. Treatment of schizophrenia in the 21st century: beyond the neurotransmitter hypothesis. Expert Rev Neurother. 2009;9:47–54. - PubMed
-
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. - PubMed
-
- Leweke FM, Schneider U, Thies M, Münte TF, Emrich HM. Effects of synthetic Δ9-tetrahydrocannabinol on binocular depth inversion of natural and artificial objects in man. Psychopharmacology. 1999;142:230–235. - PubMed
-
- D'Souza CD, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu Y-T, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

