Analysis of centrosome localization of BRCA1 and its activity in suppressing centrosomal aster formation
- PMID: 22833046
- PMCID: PMC3419063
- DOI: 10.4161/cc.21396
Analysis of centrosome localization of BRCA1 and its activity in suppressing centrosomal aster formation
Abstract
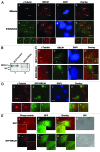
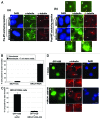
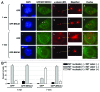
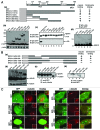
BRCA1, a product of a familial breast and ovarian cancer susceptibility gene, localizes to centrosomes and physically interacts with γ-tubulin, a key centrosomal protein for microtubule nucleation and anchoring at centrosomes. Here, we performed a rigorous analysis of centrosome localization of BRCA1, and found that BRCA1 is specifically associated with mother centrioles in unduplicated centrosomes, and daughter centrioles acquire BRCA1 prior to initiation of duplication, and thus duplicated centrosomes are both bound by BRCA1. We further found that BRCA1 suppresses centrosomal aster formation. In addition, we identified a new domain of BRCA1 critical for γ-tubulin binding, which confers not only its localization to centrosomes, but also its activity to suppress centrosomal aster formation.
Figures


Similar articles
-
Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination.Mol Cell Biol. 2005 Oct;25(19):8656-68. doi: 10.1128/MCB.25.19.8656-8668.2005. Mol Cell Biol. 2005. PMID: 16166645 Free PMC article.
-
Characterization of BRCA1 protein targeting, dynamics, and function at the centrosome: a role for the nuclear export signal, CRM1, and Aurora A kinase.J Biol Chem. 2012 Mar 2;287(10):7701-16. doi: 10.1074/jbc.M111.327296. Epub 2012 Jan 18. J Biol Chem. 2012. PMID: 22262852 Free PMC article.
-
RACK1 regulates centriole duplication by controlling localization of BRCA1 to the centrosome in mammary tissue-derived cells.Oncogene. 2019 Apr;38(16):3077-3092. doi: 10.1038/s41388-018-0647-8. Epub 2019 Jan 7. Oncogene. 2019. PMID: 30617304
-
gamma-tubulin complexes: binding to the centrosome, regulation and microtubule nucleation.Curr Opin Cell Biol. 2000 Feb;12(1):113-8. doi: 10.1016/s0955-0674(99)00064-2. Curr Opin Cell Biol. 2000. PMID: 10679351 Review.
-
The BRCA1-dependent ubiquitin ligase, gamma-tubulin, and centrosomes.Environ Mol Mutagen. 2009 Oct;50(8):649-53. doi: 10.1002/em.20475. Environ Mol Mutagen. 2009. PMID: 19274767 Review.
Cited by
-
Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro.PLoS One. 2014 Mar 3;9(3):e90332. doi: 10.1371/journal.pone.0090332. eCollection 2014. PLoS One. 2014. PMID: 24594937 Free PMC article.
-
DNA Replication Stress and Chromosomal Instability: Dangerous Liaisons.Genes (Basel). 2020 Jun 10;11(6):642. doi: 10.3390/genes11060642. Genes (Basel). 2020. PMID: 32532049 Free PMC article. Review.
-
Interaction between DNA Polymerase β and BRCA1.PLoS One. 2013 Jun 27;8(6):e66801. doi: 10.1371/journal.pone.0066801. Print 2013. PLoS One. 2013. PMID: 23826138 Free PMC article.
-
BRCA1 transports the DNA damage signal for CDDP-induced centrosome amplification through the centrosomal Aurora A.Cancer Sci. 2022 Dec;113(12):4230-4243. doi: 10.1111/cas.15573. Epub 2022 Sep 21. Cancer Sci. 2022. PMID: 36082621 Free PMC article.
-
The BRCA1 Breast Cancer Suppressor: Regulation of Transport, Dynamics, and Function at Multiple Subcellular Locations.Scientifica (Cairo). 2012;2012:796808. doi: 10.6064/2012/796808. Epub 2012 Oct 18. Scientifica (Cairo). 2012. PMID: 24278741 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
