Tolerance to MHC class II disparate allografts through genetic modification of bone marrow
- PMID: 22833118
- PMCID: PMC3651743
- DOI: 10.1038/gt.2012.57
Tolerance to MHC class II disparate allografts through genetic modification of bone marrow
Abstract
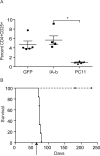
Induction of molecular chimerism through genetic modification of bone marrow is a powerful tool for the induction of tolerance. Here, we demonstrate for the first time that expression of an allogeneic MHC class II gene in autologous bone marrow cells, resulting in a state of molecular chimerism, induces tolerance to MHC class II mismatched skin grafts, a stringent test of transplant tolerance. Reconstitution of recipients with syngeneic bone marrow transduced with retrovirus encoding H-2I-A(b) (I-A(b)) resulted the long-term expression of the retroviral gene product on the surface of MHC class II-expressing bone marrow-derived cell types. Mechanistically, tolerance was maintained by the presence of regulatory T cells, which prevented proliferation and cytokine production by alloreactive host T cells. Thus, the introduction of MHC class II genes into bone marrow-derived cells through genetic engineering results in tolerance. These results have the potential to extend the clinical applicability of molecular chimerism for tolerance induction.
Figures





Similar articles
-
The importance of MHC class II in allogeneic bone marrow transplantation and chimerism-based solid organ tolerance in a rat model.PLoS One. 2020 May 22;15(5):e0233497. doi: 10.1371/journal.pone.0233497. eCollection 2020. PLoS One. 2020. PMID: 32442182 Free PMC article.
-
Induction of alloreactive CD4 T cell tolerance in molecular chimeras: a possible role for regulatory T cells.J Immunol. 2006 Mar 15;176(6):3410-6. doi: 10.4049/jimmunol.176.6.3410. J Immunol. 2006. PMID: 16517709
-
Donor MHC class II antigen is essential for induction of transplantation tolerance by bone marrow cells.J Immunol. 2000 May 1;164(9):4452-7. doi: 10.4049/jimmunol.164.9.4452. J Immunol. 2000. PMID: 10779744
-
Induction of specific tolerance to MHC-disparate allografts through genetic engineering.Exp Nephrol. 1993 Mar-Apr;1(2):128-33. Exp Nephrol. 1993. PMID: 8081957 Review.
-
Mechanistic and therapeutic role of regulatory T cells in tolerance through mixed chimerism.Curr Opin Organ Transplant. 2010 Dec;15(6):725-30. doi: 10.1097/MOT.0b013e3283401755. Curr Opin Organ Transplant. 2010. PMID: 20881493 Review.
Cited by
-
The importance of MHC class II in allogeneic bone marrow transplantation and chimerism-based solid organ tolerance in a rat model.PLoS One. 2020 May 22;15(5):e0233497. doi: 10.1371/journal.pone.0233497. eCollection 2020. PLoS One. 2020. PMID: 32442182 Free PMC article.
-
Development of Gene Transfer for Induction of Antigen-specific Tolerance.Mol Ther Methods Clin Dev. 2014 Apr 30;1:14013. doi: 10.1038/mtm.2014.13. Mol Ther Methods Clin Dev. 2014. PMID: 25558460 Free PMC article.
-
The Immune Response to the fVIII Gene Therapy in Preclinical Models.Front Immunol. 2020 Apr 15;11:494. doi: 10.3389/fimmu.2020.00494. eCollection 2020. Front Immunol. 2020. PMID: 32351497 Free PMC article. Review.
-
Transplant genetics and genomics.Nat Rev Genet. 2017 May;18(5):309-326. doi: 10.1038/nrg.2017.12. Epub 2017 Mar 13. Nat Rev Genet. 2017. PMID: 28286337 Review.
-
Molecular chimerism in IgE-mediated allergy: B-and T-cell tolerance toward highly immunogenic exogenous antigens.Chimerism. 2013 Jan-Mar;4(1):29-31. doi: 10.4161/chim.24071. Chimerism. 2013. PMID: 23712851 Free PMC article.
References
-
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–6. - PubMed
-
- Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–70. - PubMed
-
- Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

