Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-κB modulation
- PMID: 22833150
- PMCID: PMC11824778
- DOI: 10.1007/s00432-012-1292-1
Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-κB modulation
Abstract
Purpose: In our previous publication, we have shown that dihydroartemisinin could significantly inhibit the growth of CML K562 cells by its anti-proliferative and inducing apoptotic effects. Given the pivotal effect of Bcr/Abl tyrosine kinase and its downstream signal factors on CML cell proliferation and survival, we extend our study to investigate the effect of DHA on Bcr/Abl and related signal factors to further illuminate the possible mechanisms of the effect of DHA on CML cells.
Methods: The expression of Bcr/Abl was analyzed with PCR and Western blotting methods at both mRNA and protein levels. Measurement of protein expression and tyrosine phosphorylation activity of Bcr/Abl, AKT, ERK1/2, NF-κB and cytochrome c were performed with Western blotting and immunoprecipitation methods. Using the activity kits analyzed the activity of caspase 9 and caspase 3.
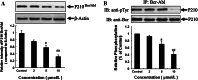
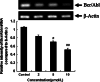
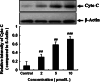
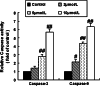
Results: The treatment with DHA results in a significant suppression on Bcr/Abl expression and leads to a concentration-dependent reduction on the Bcr/Abl tyrosine activity. Moreover, it also results in a strong influence on the downstream signal factors of Bcr/Abl, which includes inhibition of tyrosine kinase activity of AKT and ERK1/2, suppression of NF-κB protein expression, promotion of the cytochrome c release and the consequential activation of caspase 3/9 in CML K562 cells.
Conclusions: Together with our previous report, our data show that the growth inhibitory effect of DHA on CML cells might be due to the influence on Bcr/Abl expression and its downstream signal factors. DHA might be a potential novel anti-CML drug candidate and worthy of further study.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures


Similar articles
-
Dihydroartemisinin inhibits the Bcr/Abl oncogene at the mRNA level in chronic myeloid leukemia sensitive or resistant to imatinib.Biomed Pharmacother. 2013 Mar;67(2):157-63. doi: 10.1016/j.biopha.2012.10.017. Epub 2012 Nov 19. Biomed Pharmacother. 2013. PMID: 23201011
-
Inhibition of 32Dp210 cells harboring T315I mutation by a novel derivative of emodin correlates with down-regulation of BCR-ABL and its downstream signaling pathways.J Cancer Res Clin Oncol. 2015 Feb;141(2):283-93. doi: 10.1007/s00432-014-1820-2. Epub 2014 Sep 14. J Cancer Res Clin Oncol. 2015. PMID: 25217883 Free PMC article.
-
Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB.J Cancer Res Clin Oncol. 2010 Jun;136(6):897-903. doi: 10.1007/s00432-009-0731-0. Epub 2009 Nov 26. J Cancer Res Clin Oncol. 2010. PMID: 19941148 Free PMC article.
-
Chronic myelogenous leukemia: molecular and cellular aspects.J Cancer Res Clin Oncol. 1998;124(12):643-60. doi: 10.1007/s004320050228. J Cancer Res Clin Oncol. 1998. PMID: 9879825 Free PMC article. Review.
-
Does presence of complex translocations involving BCR::ABL1 in chronic myeloid leukemia affect the response rate to tyrosine kinase inhibitors? A systematic review of the literature.Ann Diagn Pathol. 2024 Aug;71:152303. doi: 10.1016/j.anndiagpath.2024.152303. Epub 2024 Apr 9. Ann Diagn Pathol. 2024. PMID: 38636337
Cited by
-
Artemisinin-type drugs for the treatment of hematological malignancies.Cancer Chemother Pharmacol. 2021 Jan;87(1):1-22. doi: 10.1007/s00280-020-04170-5. Epub 2020 Nov 3. Cancer Chemother Pharmacol. 2021. PMID: 33141328 Review.
-
A screening-based approach to circumvent tumor microenvironment-driven intrinsic resistance to BCR-ABL+ inhibitors in Ph+ acute lymphoblastic leukemia.J Biomol Screen. 2014 Jan;19(1):158-67. doi: 10.1177/1087057113501081. Epub 2013 Aug 29. J Biomol Screen. 2014. PMID: 23989453 Free PMC article.
-
Galangin suppresses human laryngeal carcinoma via modulation of caspase-3 and AKT signaling pathways.Oncol Rep. 2017 Aug;38(2):703-714. doi: 10.3892/or.2017.5767. Epub 2017 Jun 29. Oncol Rep. 2017. PMID: 28677816 Free PMC article.
-
The Potential Roles of Artemisinin and Its Derivatives in the Treatment of Type 2 Diabetes Mellitus.Front Pharmacol. 2020 Nov 26;11:585487. doi: 10.3389/fphar.2020.585487. eCollection 2020. Front Pharmacol. 2020. PMID: 33381036 Free PMC article.
-
Dihydroartemisinin Induces Apoptosis in Human Bladder Cancer Cell Lines Through Reactive Oxygen Species, Mitochondrial Membrane Potential, and Cytochrome C Pathway.Int J Prev Med. 2017 Oct 5;8:78. doi: 10.4103/ijpvm.IJPVM_258_17. eCollection 2017. Int J Prev Med. 2017. PMID: 29114376 Free PMC article.
References
-
- Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, Bhalla K (1998) Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood 91(5):1700–1705 - PubMed
-
- Benakis A, Paris M, Loutan L, Plessas CT, Plessas ST (1997) Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am J Trop Med Hyg 56(1):17–23 - PubMed
-
- Cilloni D, Saglio G (2012) Molecular pathways: BCR-ABL. Clin Cancer Res 18(4):930–937. doi:10.1158/1078-0432.CCR-10-1613 - PubMed
-
- Daley GQ, Van Etten RA, Baltimore D (1990) Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247(4944):824–830 - PubMed
-
- Disbrow GL, Baege AC, Kierpiec KA, Yuan H, Centeno JA, Thibodeaux CA, Hartmann D, Schlegel R (2005) Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res 65(23):10854–10861. doi:10.1158/0008-5472.CAN-05-1216 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

