Recent advances in the development of new transgenic animal technology
- PMID: 22833168
- PMCID: PMC11113483
- DOI: 10.1007/s00018-012-1081-7
Recent advances in the development of new transgenic animal technology
Abstract
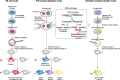
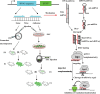
Transgenic animal technology is one of the fastest growing biotechnology areas. It is used to integrate exogenous genes into the animal genome by genetic engineering technology so that these genes can be inherited and expressed by offspring. The transgenic efficiency and precise control of gene expression are the key limiting factors in the production of transgenic animals. A variety of transgenic technologies are available. Each has its own advantages and disadvantages and needs further study because of unresolved technical and safety issues. Further studies will allow transgenic technology to explore gene function, animal genetic improvement, bioreactors, animal disease models, and organ transplantation. This article reviews the recently developed animal transgenic technologies, including the germ line stem cell-mediated method to improve efficiency, gene targeting to improve accuracy, RNA interference-mediated gene silencing technology, zinc-finger nuclease gene targeting technology and induced pluripotent stem cell technology. These new transgenic techniques can provide a better platform to develop transgenic animals for breeding new animal varieties and promote the development of medical sciences, livestock production, and other fields.
Figures



Similar articles
-
[New advances in animal transgenic technology].Yi Chuan. 2010 Jun;32(6):539-47. doi: 10.3724/sp.j.1005.2010.00539. Yi Chuan. 2010. PMID: 20566456 Review. Chinese.
-
Application of inducible and targeted gene strategies to produce transgenic fish: a review.Mar Biotechnol (NY). 2004 Mar-Apr;6(2):118-27. doi: 10.1007/s10126-003-0013-9. Mar Biotechnol (NY). 2004. PMID: 15085411 Review.
-
From Gene Targeting to Genome Editing: Transgenic animals applications and beyond.An Acad Bras Cienc. 2015 Aug;87(2 Suppl):1323-48. doi: 10.1590/0001-3765201520140710. An Acad Bras Cienc. 2015. PMID: 26397828 Review.
-
[An update on the development of transgenic animal technology].Yi Chuan. 2011 May;33(5):449-58. doi: 10.3724/sp.j.1005.2011.00449. Yi Chuan. 2011. PMID: 21586392 Review. Chinese.
-
Making better transgenic models: conditional, temporal, and spatial approaches.Mol Biotechnol. 2005 Feb;29(2):153-63. doi: 10.1385/MB:29:2:153. Mol Biotechnol. 2005. PMID: 15699570 Review.
Cited by
-
Potent and selective inhibition of pathogenic viruses by engineered ubiquitin variants.PLoS Pathog. 2017 May 18;13(5):e1006372. doi: 10.1371/journal.ppat.1006372. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542609 Free PMC article.
-
Constitutive and conditional gene knockout mice for the study of intervertebral disc degeneration: Current status, decision considerations, and future possibilities.JOR Spine. 2023 Jan 7;6(1):e1242. doi: 10.1002/jsp2.1242. eCollection 2023 Mar. JOR Spine. 2023. PMID: 36994464 Free PMC article. Review.
-
Genetic characteristics of polycistronic system‑mediated randomly‑inserted multi‑transgenes in miniature pigs and mice.Mol Med Rep. 2018 Jan;17(1):37-50. doi: 10.3892/mmr.2017.7842. Epub 2017 Oct 20. Mol Med Rep. 2018. PMID: 29115474 Free PMC article.
-
Cryopreservation Strategies for Poultry Semen: A Comprehensive Review of Techniques and Applications.Vet Sci. 2025 Feb 8;12(2):145. doi: 10.3390/vetsci12020145. Vet Sci. 2025. PMID: 40005904 Free PMC article. Review.
-
An Event-Specific Real-Time PCR Method for Measuring Transgenic Lysozyme Goat Content in Trace Samples.Foods. 2021 Apr 23;10(5):925. doi: 10.3390/foods10050925. Foods. 2021. PMID: 33922422 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

