Effects of omega-3 fatty acids on progestin stimulation of invasive properties in breast cancer
- PMID: 22833172
- PMCID: PMC10358024
- DOI: 10.1007/s12672-012-0118-6
Effects of omega-3 fatty acids on progestin stimulation of invasive properties in breast cancer
Abstract
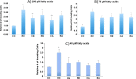
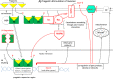
Clinical studies have shown that progestins increase breast cancer risk in hormone replacement therapy, while we and others have previously reported that progestins stimulate invasive properties in progesterone receptor (PR)-rich human breast cancer cell lines. Based on others' reports that omega-3 fatty acids inhibit metastatic properties of breast cancer, we have reviewed the literature for possible connections between omega-3 fatty-acid-driven pathways and progestin-stimulated pathways in an attempt to suggest theoretical mechanisms for possible omega-3 fatty acid inhibition of progestin stimulation of breast cancer invasion. We also present some data suggesting that fatty acids regulate progestin stimulation of invasive properties in PR-rich T47D human breast cancer cells, and that an appropriate concentration of the omega-3 fatty acid eicosapentaenoic acid inhibits progestin stimulation of invasive properties. It is hoped that focus on the inter-relationship between pathways by which omega-3 fatty acids inhibit and progestins stimulate breast cancer invasive properties will lead to further in vitro, in vivo, and clinical studies testing the hypothesis that omega-3 fatty acids can inhibit progestin stimulation of invasive properties in breast cancer, and ameliorate harmful effects of progestins which occur in combined progestin-estrogen hormone replacement therapy.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
The estrogenic activity of synthetic progestins used in oral contraceptives enhances fatty acid synthase-dependent breast cancer cell proliferation and survival.Int J Oncol. 2005 Jun;26(6):1507-15. Int J Oncol. 2005. PMID: 15870863
-
Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells.Biochem Biophys Res Commun. 2000 Nov 2;277(3):650-4. doi: 10.1006/bbrc.2000.3728. Biochem Biophys Res Commun. 2000. PMID: 11062008
-
Progestins and menopause: epidemiological studies of risks of endometrial and breast cancer.Steroids. 2000 Oct-Nov;65(10-11):659-64. doi: 10.1016/s0039-128x(00)00122-7. Steroids. 2000. PMID: 11108873
-
Progesterone receptors--animal models and cell signalling in breast cancer. Implications for breast cancer of inclusion of progestins in hormone replacement therapies.Breast Cancer Res. 2002;4(6):244-8. doi: 10.1186/bcr540. Epub 2002 Oct 7. Breast Cancer Res. 2002. PMID: 12473171 Free PMC article. Review.
-
Exogenous progestins and breast cancer.Epidemiol Rev. 1993;15(1):98-107. doi: 10.1093/oxfordjournals.epirev.a036120. Epidemiol Rev. 1993. PMID: 8405216 Review.
Cited by
-
Omega-3 polyunsaturated Fatty acids as potential chemopreventive agent for gastrointestinal cancer.J Cancer Prev. 2013 Sep;18(3):201-8. doi: 10.15430/jcp.2013.18.3.201. J Cancer Prev. 2013. PMID: 25337547 Free PMC article. Review.
-
Omega-3 Polyunsaturated Fatty Acids Intake to Regulate Helicobacter pylori-Associated Gastric Diseases as Nonantimicrobial Dietary Approach.Biomed Res Int. 2015;2015:712363. doi: 10.1155/2015/712363. Epub 2015 Aug 3. Biomed Res Int. 2015. PMID: 26339635 Free PMC article. Review.
-
Emerging targets in lipid-based therapy.Biochem Pharmacol. 2013 Mar 1;85(5):673-688. doi: 10.1016/j.bcp.2012.11.028. Epub 2012 Dec 20. Biochem Pharmacol. 2013. PMID: 23261527 Free PMC article. Review.
-
Modulation of Breast Cancer Risk Biomarkers by High-Dose Omega-3 Fatty Acids: Phase II Pilot Study in Postmenopausal Women.Cancer Prev Res (Phila). 2015 Oct;8(10):922-31. doi: 10.1158/1940-6207.CAPR-14-0336. Epub 2015 Aug 14. Cancer Prev Res (Phila). 2015. PMID: 26276744 Free PMC article. Clinical Trial.
-
Modulation of Breast Cancer Risk Biomarkers by High-Dose Omega-3 Fatty Acids: Phase II Pilot Study in Premenopausal Women.Cancer Prev Res (Phila). 2015 Oct;8(10):912-21. doi: 10.1158/1940-6207.CAPR-14-0335. Cancer Prev Res (Phila). 2015. PMID: 26438592 Free PMC article. Clinical Trial.
References
-
- American Cancer Society . Cancer facts and figures 2010. Atlanta: American Cancer Society; 2010.
-
- World Cancer Report (2008) International Agency for Research on Cancer
-
- Hissom JR, Moore MR. Progestin effects on growth in the human breast cancer cell line T47D-possible therapeutic implications. Biochem Biophys Res Commun. 1987;145:706–711. - PubMed
-
- Moore MR, Hagley RD, Hissom JR. Progestin effects on lactate dehydrogenase and growth in the human breast cancer cell line T47D. In: Hankins HD, Puett D, editors. Hormones, cell biology and cancer, potentials. New York: Alan R. Liss, Inc; 1988. pp. 161–179. - PubMed
-
- Hissom JR, Bowden RT, Moore MR. Effects of progestins, estrogens and antihormones on growth and lactate dehydrogenase in the human breast cancer cell line T47D. Endocrinology. 1989;125:418–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

