Stage-specific breast cancer incidence rates among participants and non-participants of a population-based mammographic screening program
- PMID: 22833199
- PMCID: PMC3751801
- DOI: 10.1007/s10549-012-2162-x
Stage-specific breast cancer incidence rates among participants and non-participants of a population-based mammographic screening program
Abstract
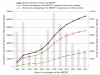
The Norwegian Breast Cancer Screening Program was rolled out county by county over the course of a decade, from 1996 to 2005, and now encompasses all Norwegian women aged 50-69 years. We aim to compare DCIS and stage-specific invasive breast cancer incidence rates among participants, non-participants, and women not yet invited to the screening program over this entire implementation period. We estimate stage-specific breast tumor incidence rates for 640,347 women 50-69 years of age invited to the screening program between 1996 and 2007. We compare incidence rates and stage distribution among women diagnosed with breast cancer who were invited and participated, invited but not participated, and women not yet invited to the screening program using two-sided Chi-squared tests to determine statistical significance between groups. The incidence of ductal carcinoma in situ (DCIS) was 3.0 times higher and invasive breast cancer was 1.5 times higher for invited participants compared to invited non-participants (p < 0.001). While the incidence of Stage I cancer was two times higher among participants compared to non-participants (p < 0.001), the incidences of Stages III and IV cancer were two and three times lower, respectively, among participants compared to non-participants (p < 0.001 for both). No significant differences in stage-specific incidence or treatment utilization rates were observed between invited non-participants and not yet invited women, except for stage IV cancers, which were detected at a higher rate among women who were not yet invited (7.5 vs. 4.6 %, p = 0.001). Compared with women invited who did not participate, participants in the screening program are more likely to be diagnosed with DCIS and early stage invasive breast cancer and are less likely to be diagnosed with advanced stage breast cancer. More research is required to determine whether these differences in stage-specific incidences among invited participants and non-participants are associated with differences in mortality rates.
Figures
Comment in
-
Comparing attendees with non-attendees in breast screening does not provide useful information about an effect on prognostic features or mortality.Breast Cancer Res Treat. 2012 Nov;136(2):617-8, author reply 619-20. doi: 10.1007/s10549-012-2250-y. Epub 2012 Sep 26. Breast Cancer Res Treat. 2012. PMID: 23011508 No abstract available.
References
-
- Vainio H, Bianchini F, editors. IARC handbook of cancer prevention Volume 7 Breast Cancer Screening. IARCPress; Lyon: [Accessed December 7, 2011]. 2002. http://www.iarc.fr.
-
- Food and Drug Administration . Quality Mammography Standards. Final Rules-21 CRF Parts 16 and 900. RIN 0910-AA24 edition. 2011. Department of Health and Human Services; Washington, DC: 1997. pp. 55925–55926. (docket No. 95N-0192)
-
- Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;151:716–236. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical