Entorhinal cortical neurons are the primary targets of FUS mislocalization and ubiquitin aggregation in FUS transgenic rats
- PMID: 22833456
- PMCID: PMC3471393
- DOI: 10.1093/hmg/dds299
Entorhinal cortical neurons are the primary targets of FUS mislocalization and ubiquitin aggregation in FUS transgenic rats
Abstract
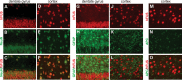
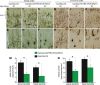
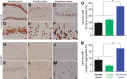
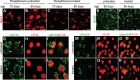
Ubiquitin-positive inclusion containing Fused in Sarcoma (FUS) defines a new subtype of frontotemporal lobar degeneration (FTLD). FTLD is characterized by progressive alteration in cognitions and it preferentially affects the superficial layers of frontotemporal cortex. Mutation of FUS is linked to amyotrophic lateral sclerosis and to motor neuron disease with FTLD. To examine FUS pathology in FTLD, we developed the first mammalian animal model expressing human FUS with pathogenic mutation and developing progressive loss of memory. In FUS transgenic rats, ubiquitin aggregation and FUS mislocalization were developed primarily in the entorhinal cortex of temporal lobe, particularly in the superficial layers of affected cortex. Overexpression of mutant FUS led to Golgi fragmentation and mitochondrion aggregation. Intriguingly, aggregated ubiquitin was not colocalized with either fragmented Golgi or aggregated mitochondria, and neurons with ubiquitin aggregates were deprived of endogenous TDP-43. Agonists of peroxisome proliferator-activated receptor gamma (PPAR-γ) possess anti-glial inflammation effects and are also shown to preserve the dendrite and dendritic spines of cortical neurons in culture. Here we show that rosiglitazone, a PPAR-γ agonist, rescued the dendrites and dendritic spines of neurons from FUS toxicity and preserved rats' spatial memory. Our FUS transgenic rats would be useful to the mechanistic study of cortical dementia in FTLD. As rosiglitazone is clinically used to treat diabetes, our results would encourage immediate application of PPAR-γ agonists in treating patients with cortical dementia.
Figures






Similar articles
-
FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration.PLoS Genet. 2011 Mar;7(3):e1002011. doi: 10.1371/journal.pgen.1002011. Epub 2011 Mar 3. PLoS Genet. 2011. PMID: 21408206 Free PMC article.
-
XBP1 depletion precedes ubiquitin aggregation and Golgi fragmentation in TDP-43 transgenic rats.J Neurochem. 2012 Nov;123(3):406-16. doi: 10.1111/jnc.12014. J Neurochem. 2012. PMID: 22970712 Free PMC article.
-
The most common type of FTLD-FUS (aFTLD-U) is associated with a distinct clinical form of frontotemporal dementia but is not related to mutations in the FUS gene.Acta Neuropathol. 2011 Jul;122(1):99-110. doi: 10.1007/s00401-011-0816-0. Epub 2011 Mar 20. Acta Neuropathol. 2011. PMID: 21424531
-
Long noncoding RNAs in TDP-43 and FUS/TLS-related frontotemporal lobar degeneration (FTLD).Neurobiol Dis. 2015 Oct;82:445-454. doi: 10.1016/j.nbd.2015.07.011. Epub 2015 Jul 26. Neurobiol Dis. 2015. PMID: 26220395 Review.
-
How do the RNA-binding proteins TDP-43 and FUS relate to amyotrophic lateral sclerosis and frontotemporal degeneration, and to each other?Curr Opin Neurol. 2012 Dec;25(6):701-7. doi: 10.1097/WCO.0b013e32835a269b. Curr Opin Neurol. 2012. PMID: 23041957 Review.
Cited by
-
Evidence of Mitochondrial Dysfunction within the Complex Genetic Etiology of Schizophrenia.Mol Neuropsychiatry. 2015 Dec;1(4):201-19. doi: 10.1159/000441252. Epub 2015 Oct 28. Mol Neuropsychiatry. 2015. PMID: 26550561 Free PMC article. Review.
-
Reactive astrocytes secrete lcn2 to promote neuron death.Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):4069-74. doi: 10.1073/pnas.1218497110. Epub 2013 Feb 19. Proc Natl Acad Sci U S A. 2013. PMID: 23431168 Free PMC article.
-
Changes in the Expression of FUS/TLS in Spinal Cords of SOD1 G93A Transgenic Mice and Correlation with Motor-Neuron Degeneration.Int J Biol Sci. 2016 Sep 14;12(10):1181-1190. doi: 10.7150/ijbs.16158. eCollection 2016. Int J Biol Sci. 2016. PMID: 27766033 Free PMC article.
-
Mutant UBQLN2P497H in motor neurons leads to ALS-like phenotypes and defective autophagy in rats.Acta Neuropathol Commun. 2018 Nov 8;6(1):122. doi: 10.1186/s40478-018-0627-9. Acta Neuropathol Commun. 2018. PMID: 30409191 Free PMC article.
-
Motor Neuron Susceptibility in ALS/FTD.Front Neurosci. 2019 Jun 27;13:532. doi: 10.3389/fnins.2019.00532. eCollection 2019. Front Neurosci. 2019. PMID: 31316328 Free PMC article. Review.
References
-
- Mackenzie I.R., Foti D., Woulfe J., Hurwitz T.A. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131:1282–1293. - PubMed
-
- Mackenzie I.R. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol. 2007;114:49–54. - PubMed
-
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

