A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma
- PMID: 22833546
- PMCID: PMC4123387
- DOI: 10.1182/blood-2012-05-425934
A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma
Abstract
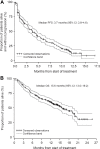
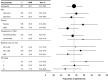
Carfilzomib is a next-generation, selective proteasome inhibitor being evaluated for the treatment of relapsed and refractory multiple myeloma. In this open-label, single-arm phase 2 study (PX-171-003-A1), patients received single-agent carfilzomib 20 mg/m(2) intravenously twice weekly for 3 of 4 weeks in cycle 1, then 27 mg/m(2) for ≤ 12 cycles. The primary endpoint was overall response rate (≥ partial response). Secondary endpoints included clinical benefit response rate (≥ minimal response), duration of response, progression-free survival, overall survival, and safety. A total of 266 patients were evaluable for safety, 257 for efficacy; 95% were refractory to their last therapy; 80% were refractory or intolerant to both bortezomib and lenalidomide. Patients had median of 5 prior lines of therapy, including bortezomib, lenalidomide, and thalidomide. Overall response rate was 23.7% with median duration of response of 7.8 months. Median overall survival was 15.6 months. Adverse events (AEs) were manageable without cumulative toxicities. Common AEs were fatigue (49%), anemia (46%), nausea (45%), and thrombocytopenia (39%). Thirty-three patients (12.4%) experienced peripheral neuropathy, primarily grades 1 or 2. Thirty-three patients (12.4%) withdrew because of an AE. Durable responses and an acceptable tolerability profile in this heavily pretreated population demonstrate the potential of carfilzomib to offer meaningful clinical benefit. This trial was registered at www.clinicaltrials.gov as #NCT00511238.
Figures
Comment in
-
Carfilzomib in multiple myeloma: gold, silver, or bronze?Blood. 2012 Oct 4;120(14):2776-7. doi: 10.1182/blood-2012-08-446534. Blood. 2012. PMID: 23043023
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26(1):73–85. - PubMed
-
- Offidani M, Leoni P, Corvatta L, et al. Outcome and toxicity in the modern era of new drugs for multiple myeloma: a reappraisal for comparison with future investigational trials. Clin Lymphoma Myeloma Leuk. 2010;10(5):353–360. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical