CIN85 phosphorylation is essential for EGFR ubiquitination and sorting into multivesicular bodies
- PMID: 22833562
- PMCID: PMC3442408
- DOI: 10.1091/mbc.E11-08-0666
CIN85 phosphorylation is essential for EGFR ubiquitination and sorting into multivesicular bodies
Abstract
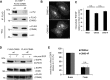
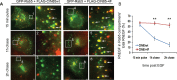
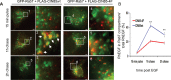
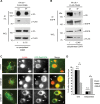
Ubiquitination of the epidermal growth factor receptor (EGFR) by cbl and its cognate adaptor cbl-interacting protein of 85 kDa (CIN85) is known to play an essential role in directing this receptor to the lysosome for degradation. The mechanisms by which this ubiquitin modification is regulated are not fully defined, nor is it clear where this process occurs. In this study we show that EGFR activation leads to a pronounced src-mediated tyrosine phosphorylation of CIN85 that subsequently influences EGFR ubiquitination. Of importance, phospho-CIN85 interacts with the Rab5-positive endosome, where it mediates the sequestration of the ubiquitinated receptor into multivesicular bodies (MVBs) for subsequent degradation. These findings provide novel insights into how src- kinase-based regulation of a cbl adaptor regulates the fate of the EGFR.
Figures


Similar articles
-
The basic amino acids in the coiled-coil domain of CIN85 regulate its interaction with c-Cbl and phosphatidic acid during epidermal growth factor receptor (EGFR) endocytosis.BMC Biochem. 2014 Jul 8;15:13. doi: 10.1186/1471-2091-15-13. BMC Biochem. 2014. PMID: 25005938 Free PMC article.
-
SH3KBP1-binding protein 1 prevents epidermal growth factor receptor degradation by the interruption of c-Cbl-CIN85 complex.Cell Biochem Funct. 2011 Oct;29(7):589-96. doi: 10.1002/cbf.1792. Epub 2011 Aug 9. Cell Biochem Funct. 2011. PMID: 21830225 Free PMC article.
-
Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis.Mol Biol Cell. 2005 Mar;16(3):1268-81. doi: 10.1091/mbc.e04-09-0832. Epub 2005 Jan 5. Mol Biol Cell. 2005. PMID: 15635092 Free PMC article.
-
[Ubiquitination-mediated degradation of epidermal growth factor receptor].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005 Feb;27(1):120-7. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005. PMID: 15782507 Review. Chinese.
-
Endocytosis and intracellular trafficking of ErbBs.Exp Cell Res. 2008 Oct 15;314(17):3093-106. doi: 10.1016/j.yexcr.2008.08.013. Epub 2008 Aug 28. Exp Cell Res. 2008. PMID: 18793634 Free PMC article. Review.
Cited by
-
The small GTPase Rab7 as a central regulator of hepatocellular lipophagy.Hepatology. 2015 Jun;61(6):1896-907. doi: 10.1002/hep.27667. Epub 2015 Apr 23. Hepatology. 2015. PMID: 25565581 Free PMC article.
-
CIN85 modulates TGFβ signaling by promoting the presentation of TGFβ receptors on the cell surface.J Cell Biol. 2015 Jul 20;210(2):319-32. doi: 10.1083/jcb.201411025. Epub 2015 Jul 13. J Cell Biol. 2015. PMID: 26169354 Free PMC article.
-
HBV secretion is regulated through the activation of endocytic and autophagic compartments mediated by Rab7 stimulation.J Cell Sci. 2015 May 1;128(9):1696-706. doi: 10.1242/jcs.158097. Epub 2015 Mar 13. J Cell Sci. 2015. PMID: 25770103 Free PMC article.
-
Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) β-Dependent Phosphorylation of GABAB1 Triggers Lysosomal Degradation of GABAB Receptors via Mind Bomb-2 (MIB2)-Mediated Lys-63-Linked Ubiquitination.Mol Neurobiol. 2019 Feb;56(2):1293-1309. doi: 10.1007/s12035-018-1142-5. Epub 2018 Jun 7. Mol Neurobiol. 2019. PMID: 29881949 Free PMC article.
-
Heregulin induces resistance to lapatinib-mediated growth inhibition of HER2-amplified cancer cells.Cancer Sci. 2013 Dec;104(12):1618-25. doi: 10.1111/cas.12290. Epub 2013 Oct 28. Cancer Sci. 2013. PMID: 24112719 Free PMC article.
References
-
- de Melker AA, van der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J Cell Sci. 2001;114:2167–2178. - PubMed
-
- Dikic I. CIN85/CMS family of adaptor molecules. FEBS Lett. 2002;529:110–115. - PubMed
-
- Ettenberg SA, Magnifico A, Cuello M, Nau MM, Rubinstein YR, Yarden Y, Weissman AM, Lipkowitz S. Cbl-b-dependent coordinated degradation of the epidermal growth factor receptor signaling complex. J Biol Chem. 2001;276:27677–27684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

