CIN85 phosphorylation is essential for EGFR ubiquitination and sorting into multivesicular bodies
- PMID: 22833562
- PMCID: PMC3442408
- DOI: 10.1091/mbc.E11-08-0666
CIN85 phosphorylation is essential for EGFR ubiquitination and sorting into multivesicular bodies
Abstract
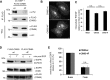
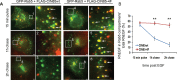
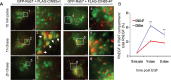
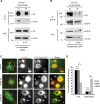
Ubiquitination of the epidermal growth factor receptor (EGFR) by cbl and its cognate adaptor cbl-interacting protein of 85 kDa (CIN85) is known to play an essential role in directing this receptor to the lysosome for degradation. The mechanisms by which this ubiquitin modification is regulated are not fully defined, nor is it clear where this process occurs. In this study we show that EGFR activation leads to a pronounced src-mediated tyrosine phosphorylation of CIN85 that subsequently influences EGFR ubiquitination. Of importance, phospho-CIN85 interacts with the Rab5-positive endosome, where it mediates the sequestration of the ubiquitinated receptor into multivesicular bodies (MVBs) for subsequent degradation. These findings provide novel insights into how src- kinase-based regulation of a cbl adaptor regulates the fate of the EGFR.
Figures


References
-
- de Melker AA, van der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J Cell Sci. 2001;114:2167–2178. - PubMed
-
- Dikic I. CIN85/CMS family of adaptor molecules. FEBS Lett. 2002;529:110–115. - PubMed
-
- Ettenberg SA, Magnifico A, Cuello M, Nau MM, Rubinstein YR, Yarden Y, Weissman AM, Lipkowitz S. Cbl-b-dependent coordinated degradation of the epidermal growth factor receptor signaling complex. J Biol Chem. 2001;276:27677–27684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

