ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation
- PMID: 22833566
- PMCID: PMC3442419
- DOI: 10.1091/mbc.E12-03-0172
ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation
Abstract
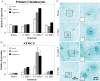
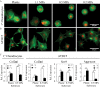
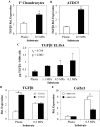
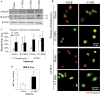
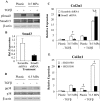
Cells encounter physical cues such as extracellular matrix (ECM) stiffness in a microenvironment replete with biochemical cues. However, the mechanisms by which cells integrate physical and biochemical cues to guide cellular decision making are not well defined. Here we investigate mechanisms by which chondrocytes generate an integrated response to ECM stiffness and transforming growth factor β (TGFβ), a potent agonist of chondrocyte differentiation. Primary murine chondrocytes and ATDC5 cells grown on 0.5-MPa substrates deposit more proteoglycan and express more Sox9, Col2α1, and aggrecan mRNA relative to cells exposed to substrates of any other stiffness. The chondroinductive effect of this discrete stiffness, which falls within the range reported for articular cartilage, requires the stiffness-sensitive induction of TGFβ1. Smad3 phosphorylation, nuclear localization, and transcriptional activity are specifically increased in cells grown on 0.5-MPa substrates. ECM stiffness also primes cells for a synergistic response, such that the combination of ECM stiffness and exogenous TGFβ induces chondrocyte gene expression more robustly than either cue alone through a p38 mitogen-activated protein kinase-dependent mechanism. In this way, the ECM stiffness primes the TGFβ pathway to efficiently promote chondrocyte differentiation. This work reveals novel mechanisms by which cells integrate physical and biochemical cues to exert a coordinated response to their unique cellular microenvironment.
Figures

References
-
- Aigner T, Kurz B, Fukui N, Sandell L. Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr Opin Rheumatol. 2002;14:578–584. - PubMed
-
- Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res. 1986;4:379–392. - PubMed
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

