High-capacity selective uptake of cholesteryl ester from native LDL during macrophage foam cell formation
- PMID: 22833685
- PMCID: PMC3435541
- DOI: 10.1194/jlr.M026534
High-capacity selective uptake of cholesteryl ester from native LDL during macrophage foam cell formation
Abstract
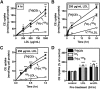
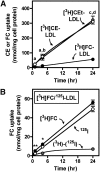
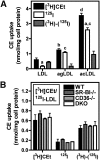
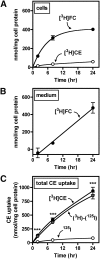
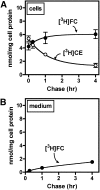
Macrophage foam cells are a defining pathologic feature of atherosclerotic lesions. Recent studies have demonstrated that at high concentrations associated with hypercholesterolemia, native LDL induces macrophage lipid accumulation. LDL particles are taken up by macrophages as part of bulk fluid pinocytosis. However, the uptake and metabolism of cholesterol from native LDL during foam cell formation has not been clearly defined. Previous reports have suggested that selective cholesteryl ester (CE) uptake might contribute to cholesterol uptake from LDL independently of particle endocytosis. In this study we demonstrate that the majority of macrophage LDL-derived cholesterol is acquired by selective CE uptake in excess of LDL pinocytosis and degradation. Macrophage selective CE uptake does not saturate at high LDL concentrations and is not down-regulated during cholesterol accumulation. In contrast to CE uptake, macrophages exhibit little selective uptake of free cholesterol (FC) from LDL. Following selective uptake from LDL, CE is rapidly hydrolyzed by a novel chloroquine-sensitive pathway. FC released from LDL-derived CE hydrolysis is largely effluxed from cells but also is subject to ACAT-mediated reesterification. These results indicate that selective CE uptake plays a major role in macrophage metabolism of LDL.
Figures



Similar articles
-
Minimally oxidized LDL inhibits macrophage selective cholesteryl ester uptake and native LDL-induced foam cell formation.J Lipid Res. 2014 Aug;55(8):1648-56. doi: 10.1194/jlr.M044644. Epub 2014 Jun 2. J Lipid Res. 2014. PMID: 24891335 Free PMC article.
-
Fluid-phase pinocytosis of native low density lipoprotein promotes murine M-CSF differentiated macrophage foam cell formation.PLoS One. 2013;8(3):e58054. doi: 10.1371/journal.pone.0058054. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23536783 Free PMC article.
-
Oxidized low density lipoprotein leads to macrophage accumulation of unesterified cholesterol as a result of lysosomal trapping of the lipoprotein hydrolyzed cholesteryl ester.J Lipid Res. 1994 May;35(5):803-19. J Lipid Res. 1994. PMID: 8071603
-
Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles.Curr Opin Lipidol. 2011 Oct;22(5):386-93. doi: 10.1097/MOL.0b013e32834adadb. Curr Opin Lipidol. 2011. PMID: 21881499 Free PMC article. Review.
-
Fluid-phase pinocytosis of LDL by macrophages: a novel target to reduce macrophage cholesterol accumulation in atherosclerotic lesions.Curr Pharm Des. 2013;19(33):5865-72. doi: 10.2174/1381612811319330005. Curr Pharm Des. 2013. PMID: 23438954 Free PMC article. Review.
Cited by
-
Quantification of sterol-specific response in human macrophages using automated imaged-based analysis.Lipids Health Dis. 2017 Dec 13;16(1):242. doi: 10.1186/s12944-017-0629-9. Lipids Health Dis. 2017. PMID: 29237459 Free PMC article.
-
Influence of Fatty Acid Modification on Uptake of Lovastatin-Loaded Reconstituted High Density Lipoprotein by Foam Cells.Pharm Res. 2018 May 7;35(7):134. doi: 10.1007/s11095-018-2419-0. Pharm Res. 2018. PMID: 29736804
-
Minimally oxidized LDL inhibits macrophage selective cholesteryl ester uptake and native LDL-induced foam cell formation.J Lipid Res. 2014 Aug;55(8):1648-56. doi: 10.1194/jlr.M044644. Epub 2014 Jun 2. J Lipid Res. 2014. PMID: 24891335 Free PMC article.
-
New developments in selective cholesteryl ester uptake.Curr Opin Lipidol. 2013 Oct;24(5):386-92. doi: 10.1097/MOL.0b013e3283638042. Curr Opin Lipidol. 2013. PMID: 23842142 Free PMC article. Review.
-
Linoleic acid suppresses cholesterol efflux and ATP-binding cassette transporters in murine bone marrow-derived macrophages.Lipids. 2014 May;49(5):415-22. doi: 10.1007/s11745-014-3890-y. Epub 2014 Mar 5. Lipids. 2014. PMID: 24595513 Free PMC article.
References
-
- 1984 The Lipid Research Clinics Coronary Primary Prevention Trial Results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. J. Am. Med. Assoc. 251: 365–374 - PubMed
-
- 1994 Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 344: 1383–1389 - PubMed
-
- Nissen S. E., Nicholls S. J., Sipahi I., Libby P., Raichlen J. S., Ballantyne C. M., Davignon J., Erbel R., Fruchart J. C., Tardif J. C., et al. 2006. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. J. Am. Med. Assoc. 295: 1556–1565 - PubMed
-
- Webb N. R., Moore K. J. 2007. Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr. Drug Targets. 8: 1249–1263 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

