In vitro endothelialization of electrospun terpolymer scaffolds: evaluation of scaffold type and cell source
- PMID: 22834688
- PMCID: PMC3530934
- DOI: 10.1089/ten.TEA.2011.0655
In vitro endothelialization of electrospun terpolymer scaffolds: evaluation of scaffold type and cell source
Abstract
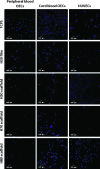
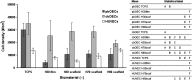
A family of methacrylic terpolymer biomaterials was electrospun into three-dimensional scaffolds. The glass transition temperature of the polymer correlates with the morphology of the resulting scaffold. Glassy materials produce scaffolds with discrete fibers and large pore areas (1531±1365 μm(2)), while rubbery materials produce scaffolds with fused fibers and smaller pore areas (154±110 μm(2)). Three different endothelial-like cell populations were seeded onto these scaffolds under static conditions: human umbilical vein endothelial cells (HUVECs), adult human peripheral blood-derived outgrowth endothelial cells, and umbilical cord blood-derived human blood outgrowth endothelial cells. Cellular behavior depended on both cell type and scaffold topography. Specifically, cord blood-derived outgrowth endothelial cells showed more robust adhesion and growth on all scaffolds in comparison to other cell types as measured by the density of adherent cells, the number of proliferative cells, and the enzymatic activity of the adherent cells. Peripheral blood-derived outgrowth cells exhibited less ability to inhabit the terpolymer interfaces in comparison to their cord blood-derived counterparts. HUVECs also exhibited less of a capacity to colonize the terpolymer interfaces in comparison to the cord blood-derived cells. However, the mature endothelial cells did show scaffold-dependent behavior. Specifically, we observed an increase in their ability to populate the low-porosity scaffolds. All cells maintained an endothelial phenotype after 1 week of culture on the electrospun scaffolds.
Figures






Similar articles
-
Electrospun scaffold topography affects endothelial cell proliferation, metabolic activity, and morphology.J Biomed Mater Res A. 2010 Sep 15;94(4):1195-204. doi: 10.1002/jbm.a.32802. J Biomed Mater Res A. 2010. PMID: 20694986
-
Novel method to improve vascularization of tissue engineered constructs with biodegradable fibers.Biofabrication. 2016 Jan 7;8(1):015004. doi: 10.1088/1758-5090/8/1/015004. Biofabrication. 2016. PMID: 26741237
-
Cell infiltration and vascularization in porous nanoyarn scaffolds prepared by dynamic liquid electrospinning.J Biomed Nanotechnol. 2014 Apr;10(4):603-14. doi: 10.1166/jbn.2014.1733. J Biomed Nanotechnol. 2014. PMID: 24734512
-
Interactions between endothelial cells and electrospun methacrylic terpolymer fibers for engineered vascular replacements.J Biomed Mater Res A. 2009 Dec 15;91(4):1131-9. doi: 10.1002/jbm.a.32276. J Biomed Mater Res A. 2009. PMID: 19148926 Free PMC article.
-
Fabrication of PU/PEGMA crosslinked hybrid scaffolds by in situ UV photopolymerization favoring human endothelial cells growth for vascular tissue engineering.J Mater Sci Mater Med. 2012 Jun;23(6):1499-510. doi: 10.1007/s10856-012-4613-7. Epub 2012 Mar 20. J Mater Sci Mater Med. 2012. PMID: 22430593
Cited by
-
Glass Transition in Crosslinked Nanocomposite Scaffolds of Gelatin/Chitosan/Hydroxyapatite.Polymers (Basel). 2019 Apr 9;11(4):642. doi: 10.3390/polym11040642. Polymers (Basel). 2019. PMID: 30970604 Free PMC article.
-
A Material Conferring Hemocompatibility.Sci Rep. 2016 Jun 6;6:26848. doi: 10.1038/srep26848. Sci Rep. 2016. PMID: 27264087 Free PMC article.
References
-
- Ratner B. Hoffman A. Schoen F. Lemons J. An introduction to materials in medicine. In: Ratner B., editor; Hoffman A., editor; Schoen F., editor; Lemons J., editor. Biomaterials Science. second. San Diego: Elsevier Academic Press; 2004.
-
- Lamba N. Woodhouse K. Cooper S. Polyurethanes in Biomedical Applications. Boca Raton: CRC Press LLC; 1998.
-
- Hench L. Biomaterials: a forecast for the future. Biomaterials. 1998;19:1419. - PubMed
-
- Bos G. Poot A. Beugeling T. van Aken W. Feijen Small diameter vascular graft prostheses: current status. Arch Phisiol Biochem. 1998;106:100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

