Electrochemical immunosensors for detection of cancer protein biomarkers
- PMID: 22835068
- PMCID: PMC3429657
- DOI: 10.1021/nn3023969
Electrochemical immunosensors for detection of cancer protein biomarkers
Abstract
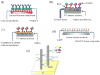
Bioanalytical methods have experienced unprecedented growth in recent years, driven in large part by the need for faster, more sensitive, more portable ("point of care") systems to detect protein biomarkers for clinical diagnosis. Electrochemical detection strategies, used in conjunction with immunosensors, offer advantages because they are fast, simple, and low cost. Recent developments in electrochemical immunosensors have significantly improved the sensitivity needed to detect low concentrations of biomarkers present in early stages of cancer. Moreover, the coupling of electrochemical devices with nanomaterials, such as gold nanoparticles, carbon nanotubes, magnetic particles, and quantum dots, offers multiplexing capability for simultaneous measurements of multiple cancer biomarkers. This review will discuss recent advances in the development of electrochemical immunosensors for the next generation of cancer diagnostics, with an emphasis on opportunities for further improvement in cancer diagnostics and treatment monitoring. Details will be given for strategies to increase sensitivity through multilabel amplification, coupled with high densities of capture molecules on sensor surfaces. Such sensors are capable of detecting a wide range of protein quantities, from nanogram to femtogram (depending on the protein biomarkers of interest), in a single sample.
Figures
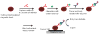





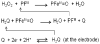

References
-
- Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, et al. Biomarkers and SurrogateEndpoints: Preferred Definitions and Conceptual Framework. Clin Pharmacol Ther. 2001;69:89–95. - PubMed
-
- Wulfkuhle JD, Liotta LA, Petricoin EF. Proteomic Applications for the Early Detection of Cancer. Nat Rev Cancer. 2003;3:267–275. - PubMed
-
- Kulasingam V, Diamandis EP. Strategies for Discovering Novel Cancer Biomarkers through Utilization of Emerging Technologies. Nat Clin Pract Oncol. 2008;5:588–599. - PubMed
-
- Ludwig JA, Weinstein JN. Biomarkers in Cancer Staging, Prognosis and TreatmentSelection. Nat Rev Cancer. 2005;5:845–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

