Relaxin induces matrix-metalloproteinases-9 and -13 via RXFP1: induction of MMP-9 involves the PI3K, ERK, Akt and PKC-ζ pathways
- PMID: 22835547
- PMCID: PMC3447121
- DOI: 10.1016/j.mce.2012.07.006
Relaxin induces matrix-metalloproteinases-9 and -13 via RXFP1: induction of MMP-9 involves the PI3K, ERK, Akt and PKC-ζ pathways
Abstract
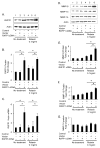
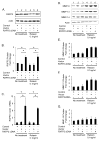
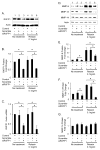
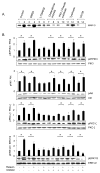
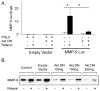
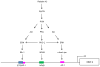
We determined the precise role of relaxin family peptide (RXFP) receptors-1 and -2 in the regulation of MMP-9 and -13 by relaxin, and delineated the signaling cascade that contributes to relaxin's modulation of MMP-9 in fibrocartilaginous cells. Relaxin treatment of cells in which RXFP1 was silenced resulted in diminished induction of MMP-9 and -13 by relaxin, whereas overexpression of RXFP1 potentiated the relaxin-induced expression of these proteinases. Suppression or overexpression of RXFP2 resulted in no changes in the relaxin-induced MMP-9 and -13. Studies using chemical inhibitors and siRNAs to signaling molecules showed that PI3K, Akt, ERK and PKC-ζ and the transcription factors Elk-1, c-fos and, to a lesser extent, NF-κB are involved in relaxin's induction of MMP-9. Our findings provide the first characterization of signaling cascade involved in the regulation of any MMP by relaxin and offer mechanistic insights on how relaxin likely mediates extracellular matrix turnover.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Figures




References
-
- Bani D. Relaxin: a pleiotropic hormone. Gen Pharmacol. 1997;28:13–22. - PubMed
-
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. Receptors for relaxin family peptides. Ann N Y Acad Sci. 2005;1041:61–76. - PubMed
-
- Bathgate RA, Samuel CS, Burazin TC, Gundlach AL, Tregear GW. Relaxin: new peptides, receptors and novel actions. Trends Endocrinol Metab. 2003;14:207–13. - PubMed
-
- Bogatcheva NV, Truong A, Feng S, Engel W, Adham IM, Agoulnik AI. GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol. 2003;17:2639–46. - PubMed
-
- Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006;281:34833–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

