Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation
- PMID: 22835669
- PMCID: PMC3424733
- DOI: 10.1016/j.jacc.2012.02.070
Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation
Abstract
Objectives: We tested whether an assessment of myocardial scarring by cardiac magnetic resonance imaging (MRI) would improve risk stratification in patients evaluated for implantable cardioverter-defibrillator (ICD) implantation.
Background: Current sudden cardiac death risk stratification emphasizes left ventricular ejection fraction (LVEF); however, most patients suffering sudden cardiac death have a preserved LVEF, and many with poor LVEF do not benefit from ICD prophylaxis.
Methods: One hundred thirty-seven patients undergoing evaluation for possible ICD placement were prospectively enrolled and underwent cardiac MRI assessment of LVEF and scar. The pre-specified primary endpoint was death or appropriate ICD discharge for sustained ventricular tachyarrhythmia.
Results: During a median follow-up of 24 months the primary endpoint occurred in 39 patients. Whereas the rate of adverse events steadily increased with decreasing LVEF, a sharp step-up was observed for scar size >5% of left ventricular mass (hazard ratio [HR]: 5.2; 95% confidence interval [CI]: 2.0 to 13.3). On multivariable Cox proportional hazards analysis, including LVEF and electrophysiological-study results, scar size (as a continuous variable or dichotomized at 5%) was an independent predictor of adverse outcome. Among patients with LVEF >30%, those with significant scarring (>5%) had higher risk than those with minimal or no (≤5%) scarring (HR: 6.3; 95% CI: 1.4 to 28.0). Those with LVEF >30% and significant scarring had risk similar to patients with LVEF ≤30% (p = 0.56). Among patients with LVEF ≤30%, those with significant scarring again had higher risk than those with minimal or no scarring (HR: 3.9; 95% CI: 1.2 to 13.1). Those with LVEF ≤30% and minimal scarring had risk similar to patients with LVEF >30% (p = 0.71).
Conclusions: Myocardial scarring detected by cardiac MRI is an independent predictor of adverse outcome in patients being considered for ICD placement. In patients with LVEF >30%, significant scarring (>5% LV) identifies a high-risk cohort similar in risk to those with LVEF ≤30%. Conversely, in patients with LVEF ≤30%, minimal or no scarring identifies a low-risk cohort similar to those with LVEF >30%.
Copyright © 2012 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
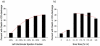



Comment in
-
Scaring myocardial scars: new targets for the electrical fairy?J Am Coll Cardiol. 2012 Jul 31;60(5):421-2. doi: 10.1016/j.jacc.2012.02.071. J Am Coll Cardiol. 2012. PMID: 22835670 No abstract available.
References
-
- American Heart Association . Heart Disease and Stroke Statistics - 2007 Update. American Heart Association; Dallas, Texas: 2007.
-
- Centers for Medicaid and Medicare Services Decision Memo for Implantable Defibrillators (CAG-00157R3). Centers for Medicaid and Medicare Services. 2007
-
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. - PubMed
-
- Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. - PubMed
-
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

