Laser-engineered dissolving microneedles for active transdermal delivery of nadroparin calcium
- PMID: 22836025
- PMCID: PMC4119960
- DOI: 10.1016/j.ejpb.2012.07.008
Laser-engineered dissolving microneedles for active transdermal delivery of nadroparin calcium
Abstract
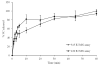
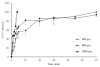
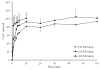
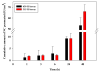
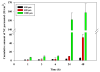
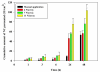
There is an urgent need to replace the injection currently used for low molecular weight heparin (LMWH) multidose therapy with a non- or minimally invasive delivery approach. In this study, laser-engineered dissolving microneedle (DMN) arrays fabricated from aqueous blends of 15% w/w poly(methylvinylether-co-maleic anhydride) were used for the first time in active transdermal delivery of the LMWH nadroparin calcium (NC). Importantly, an array loading of 630IU of NC was achieved without compromising the array mechanical strength or drug bioactivity. Application of NC-DMNs to dermatomed human skin (DHS) using the single-step 'poke and release' approach allowed permeation of approximately 10.6% of the total NC load over a 48-h study period. The cumulative amount of NC that permeated DHS at 24h and 48h attained 12.28±4.23IU/cm(2) and 164.84±8.47IU/cm(2), respectively. Skin permeation of NC could be modulated by controlling the DMN array variables, such as MN length and array density as well as application force to meet various clinical requirements including adjustment for body mass and renal function. NC-loaded DMN offers great potential as a relatively low-cost functional delivery system for enhanced transdermal delivery of LMWH and other macromolecules.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures




References
-
- Harrison L, McGinnis J, Crowther M, Ginsberg J, Hirsh J. Assessment of outpatient treatment of deep-vein thrombosis with low-molecular-weight heparin. Arch. Intern. Med. 1998;158:2001–2003. - PubMed
-
- Hyers TM, Agnelli G, Hull RD, Weg JG, Morris TA, Samama M, Tapson V. Antithrombotic therapy for venous thromboembolic disease. Chest. 1998;114:561S–578S. - PubMed
-
- Song YK, Kim CK. Topical delivery of low-molecular-weight heparin with surface-charged flexible liposomes. Biomaterials. 2006;27:271–280. - PubMed
-
- Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S–94S. - PubMed
-
- Carter CJ, Kelton JG, Hirsh J, Cerskus A, Santos AV, Gent M. The relationship between the hemorrhagic and antithrombotic properties of low molecular weight heparin in rabbits. Blood. 1982;59:1239–1245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

