Toward a mouse model of hind limb ischemia to test therapeutic angiogenesis
- PMID: 22836102
- PMCID: PMC3508332
- DOI: 10.1016/j.jvs.2012.04.067
Toward a mouse model of hind limb ischemia to test therapeutic angiogenesis
Abstract
Introduction: Several clinical trials are currently evaluating stem cell therapy for patients with critical limb ischemia that have no other surgical or endovascular options for revascularization. However, these trials are conducted with different protocols, including use of different stem cell populations and different injection protocols, providing little means to compare trials and guide therapy. Accordingly, we developed a murine model of severe ischemia to allow methodic testing of relevant clinical parameters.
Methods: High femoral artery ligation and total excision of the superficial femoral artery was performed on C57BL/6 mice. Mononuclear cells (MNCs) were isolated from the bone marrow of donor mice, characterized using fluorescence-activated cell sorting, and injected (5×10(5) to 2×10(6)) into the semimembranosus (proximal) or gastrocnemius (distal) muscle. Vascular and functional outcomes were measured using invasive Doppler imaging, laser Doppler perfusion imaging, and the Tarlov and ischemia scores. Histologic analysis included quantification of muscle fiber area and number as well as capillary density.
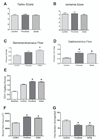
Results: Blood flow and functional outcomes were improved in MNC-treated mice compared with controls over 28 days (flow: P<.0001; Tarlov: P=.0004; ischemia score: P=.0002). MNC-treated mice also showed greater gastrocnemius fiber area (P=.0053) and increased capillary density (P=.0004). Dose-response analysis showed increased angiogenesis and gastrocnemius fiber area but no changes in macroscopic vascular flow or functional scores. Overall functional outcomes in mice injected proximally to the ischemic area were similar to mice injected more distally, but muscle flow, capillary density, and gastrocnemius fiber area were increased (P<.05).
Conclusions: High femoral ligation with complete excision of the superficial femoral artery is a reliable model of severe hind limb ischemia in C57BL/6 mice that shows a response to MNC treatment for functional and vascular outcomes. A dose response to the injection of MNCs appears to be present, at least microscopically, suggesting that an optimal cell number for stem cell therapy exists and that preclinical testing needs to be performed to optimally guide human trials. Injection of MNCs proximal to the site of ischemia may provide different outcomes compared with distal injection and warrants additional study.
Published by Mosby, Inc.
Figures




References
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. - PubMed
-
- Sprengers RW, Lips DJ, Moll FL, et al. Progenitor cell therapy in patients with critical limb ischemia without surgical options. Ann Surg. 2008;247(3):411–420. - PubMed
-
- Minar E. Critical limb ischaemia. Hamostaseologie. 2009;29(1):102–109. - PubMed
-
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

