α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis
- PMID: 22836248
- PMCID: PMC3426499
- DOI: 10.1523/JNEUROSCI.0535-12.2012
α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis
Abstract
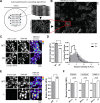
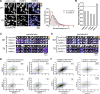
Although the presynaptic protein α-synuclein is a recognized player in neurodegeneration, its precise physiologic function(s) and/or role in human disease remains unclear. An emerging consensus from previous studies in lower-order systems is that α-synuclein interferes with vesicle-trafficking pathways; however putative neuronal correlates are unknown. Here we explore consequences of α-synuclein modulation in cultured mouse hippocampal neurons; coupling α-synuclein overexpression and knock-out model-systems with contemporary imaging paradigms. Our studies reveal an unexpected role of α-synuclein in attenuating the mobility of recycling pool (RP) vesicles between presynaptic boutons--called "superpool" trafficking--and also in maintaining the overall size of RPs at synapses. While an excess of α-synuclein led to smaller RPs and inhibited intersynaptic trafficking, an absence of α-synuclein triggered converse changes with larger RPs and enhanced intersynaptic trafficking. The data collectively suggest a model where α-synuclein maintains RP homeostasis by modulating intersynaptic vesicular dynamics, and provide a putative neuronal correlate of α-synuclein-induced impairments in vesicle-trafficking previously reported in lower-order systems.
Figures


References
-
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31:7264–7274. - PMC - PubMed
-
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. - PMC - PubMed
-
- Darcy KJ, Staras K, Collinson LM, Goda Y. Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat Neurosci. 2006;9:315–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
