B-Raf activation cooperates with PTEN loss to drive c-Myc expression in advanced prostate cancer
- PMID: 22836754
- PMCID: PMC3445712
- DOI: 10.1158/0008-5472.CAN-12-0820
B-Raf activation cooperates with PTEN loss to drive c-Myc expression in advanced prostate cancer
Abstract
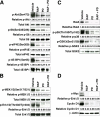
Both the PI3K → Akt → mTOR and mitogen-activated protein kinase (MAPK) signaling pathways are often deregulated in prostate tumors with poor prognosis. Here we describe a new genetically engineered mouse model of prostate cancer in which PI3K-Akt-mTOR signaling is activated by inducible disruption of PTEN, and extracellular signal-regulated kinase 1/2 (ERK1/2) MAPK signaling is activated by inducible expression of a BRAF(V600E) oncogene. These tissue-specific compound mutant mice develop lethal prostate tumors that are inherently resistant to castration. These tumors bypass cellular senescence and disseminate to lymph nodes, bone marrow, and lungs where they form overt metastases in approximately 30% of the cases. Activation of PI3K → Akt → mTOR and MAPK signaling pathways in these prostate tumors cooperate to upregulate c-Myc. Accordingly, therapeutic treatments with rapamycin and PD0325901 to target these pathways, respectively, attenuate c-Myc levels and reduce tumor and metastatic burden. Together, our findings suggest a generalized therapeutic approach to target c-Myc activation in prostate cancer by combinatorial targeting of the PI3K → Akt → mTOR and ERK1/2 MAPK signaling pathways.
Figures

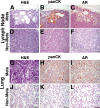
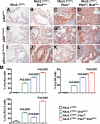

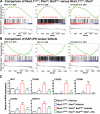
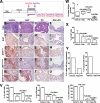
References
-
- Jenkins RB, Qian J, Lieber MM, Bostwick DG. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 1997;57:524–31. - PubMed
-
- Sato K, Qian J, Slezak JM, Lieber MM, Bostwick DG, Bergstralh EJ, et al. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. Journal of the National Cancer Institute. 1999;91:1574–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA131261/CA/NCI NIH HHS/United States
- U01 CA084294/CA/NCI NIH HHS/United States
- R01 CA173481/CA/NCI NIH HHS/United States
- U01 CA168426/CA/NCI NIH HHS/United States
- R01 CA115717/CA/NCI NIH HHS/United States
- DK076602/DK/NIDDK NIH HHS/United States
- U01 CA105490/CA/NCI NIH HHS/United States
- U54 CA121852/CA/NCI NIH HHS/United States
- CA115717/CA/NCI NIH HHS/United States
- CA154293/CA/NCI NIH HHS/United States
- R01 DK076602/DK/NIDDK NIH HHS/United States
- CA084294/CA/NCI NIH HHS/United States
- P01 CA154293/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

