P2X4R+ microglia drive neuropathic pain
- PMID: 22837036
- PMCID: PMC5023423
- DOI: 10.1038/nn.3155
P2X4R+ microglia drive neuropathic pain
Abstract
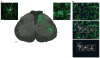
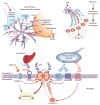
Neuropathic pain, the most debilitating of all clinical pain syndromes, may be a consequence of trauma, infection or pathology from diseases that affect peripheral nerves. Here we provide a framework for understanding the spinal mechanisms of neuropathic pain as distinct from those of acute pain or inflammatory pain. Recent work suggests that a specific microglia response phenotype characterized by de novo expression of the purinergic receptor P2X4 is critical for the pathogenesis of pain hypersensitivity caused by injury to peripheral nerves. Stimulating P2X4 receptors initiates a core pain signaling pathway mediated by release of brain-derived neurotrophic factor, which produces a disinhibitory increase in intracellular chloride in nociceptive (pain-transmitting) neurons in the spinal dorsal horn. The changes caused by signaling from P2X4R(+) microglia to nociceptive transmission neurons may account for the main symptoms of neuropathic pain in humans, and they point to specific interventions to alleviate this debilitating condition.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. - PubMed
-
- Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. - PubMed
-
- Nissl F. Ueber einige Beziehungen zur Nervenzellenerkrankungen und gliosen Erscheinungen bei verschiedenen Psychosen. Arch Psychiatr. 1899;32:656–676.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

