Phase IIa study of the immunogenicity and safety of the novel Staphylococcus aureus vaccine V710 in adults with end-stage renal disease receiving hemodialysis
- PMID: 22837094
- PMCID: PMC3428394
- DOI: 10.1128/CVI.00034-12
Phase IIa study of the immunogenicity and safety of the novel Staphylococcus aureus vaccine V710 in adults with end-stage renal disease receiving hemodialysis
Abstract
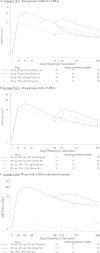
Bacteremia is the second leading cause of death in patients with end-stage renal disease who are on hemodialysis. A vaccine eliciting long-term immune responses against Staphylococcus aureus in patients on chronic hemodialysis may reduce the incidence of bacteremia and its complications in these patients. V710 is a vaccine containing iron surface determinant B (IsdB), a highly conserved S. aureus surface protein, which has been shown to be immunogenic in healthy subjects. In this blinded phase II immunogenicity study, 206 chronic hemodialysis patients between the ages of 18 and 80 years old were randomized to receive 60 μg V710 (with or without adjuvant), 90 μg V710 (with adjuvant), or a placebo in various combinations on days 1, 28, and 180. All 201 vaccinated patients were to be followed through day 360. The primary hypothesis was that at least 1 of the 3 groups receiving 2 V710 doses on days 1 and 28 would have a ≥2.5 geometric mean fold rise (GMFR) in anti-IsdB IgG titers over the baseline 28 days after the second vaccination (day 56). At day 56, all three groups receiving 2 doses of V710 achieved a ≥2.5 GMFR in anti-IsdB antibodies compared to the baseline (P values of <0.001 for all 3 groups), satisfying the primary immunogenicity hypothesis. None of the 33 reported serious adverse experiences were considered vaccine related by the investigators. V710 induced sustained antibody responses for at least 1 year postvaccination in patients on chronic hemodialysis.
Figures


References
-
- Aronoff GR, Maxwell DR, Batteiger BE, Fineberg NS. 1985. Hepatitis B virus vaccine: a randomized trial of a reduced dose regimen in hemodialysis patients. Am. J. Kidney Dis. 6:170–172 - PubMed
-
- Centers for Disease Control 2007. Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients-United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 56:197–199 - PubMed
-
- Engemann JJ, et al. 2005. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect. Control Hosp. Epidemiol. 26:534–539 - PubMed
-
- Fowler VG, et al. 2012. Efficacy and safety of V710, a Staphylococcus aureus vaccine, in preventing bacteraemia and/or deep sternal wound infections in patients undergoing cardiac surgery, abstr O164. Abstr. 22nd Eur. Congr. Infect. Dis. Clin. Microbiol., London, United Kingdom; 31 March to 3 April 2012. http://onlinelibrary.wiley.com/doi/10.1111/j.1469-0691.2012.03801.x/pdf - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

