A DNA-based candidate HIV vaccine delivered via in vivo electroporation induces CD4 responses toward the α4β7-binding V2 loop of HIV gp120 in healthy volunteers
- PMID: 22837097
- PMCID: PMC3428404
- DOI: 10.1128/CVI.00327-12
A DNA-based candidate HIV vaccine delivered via in vivo electroporation induces CD4 responses toward the α4β7-binding V2 loop of HIV gp120 in healthy volunteers
Abstract
Administration of a clade C/B' candidate HIV-1 DNA vaccine, ADVAX, by in vivo electroporation (EP) was safe and more immunogenic than intramuscular administration without EP. The breadth and specificity of T-cell responses to full-length Env were mapped. Responses to multiple Env regions were induced, with most focusing on V3/C4 and V2 regions, including the α4β7 integrin-binding domain. The breadth of responses induced by this DNA vaccine regimen was comparable to that of viral-vectored vaccine regimens.
Figures
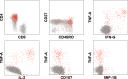
Similar articles
-
Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation.Vaccine. 2011 Jan 17;29(4):795-803. doi: 10.1016/j.vaccine.2010.11.011. Epub 2010 Nov 19. Vaccine. 2011. PMID: 21094270
-
In vivo electroporation in DNA-VLP prime-boost preferentially enhances HIV-1 envelope-specific IgG2a, neutralizing antibody and CD8 T cell responses.Vaccine. 2017 Apr 11;35(16):2042-2051. doi: 10.1016/j.vaccine.2017.03.006. Epub 2017 Mar 17. Vaccine. 2017. PMID: 28318765
-
In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers.PLoS One. 2011;6(5):e19252. doi: 10.1371/journal.pone.0019252. Epub 2011 May 16. PLoS One. 2011. PMID: 21603651 Free PMC article. Clinical Trial.
-
Electroporation-mediated administration of candidate DNA vaccines against HIV-1.Methods Mol Biol. 2014;1121:291-307. doi: 10.1007/978-1-4614-9632-8_26. Methods Mol Biol. 2014. PMID: 24510833 Review.
-
Electroporation delivery of DNA vaccines: prospects for success.Curr Opin Immunol. 2011 Jun;23(3):421-9. doi: 10.1016/j.coi.2011.03.008. Epub 2011 Apr 27. Curr Opin Immunol. 2011. PMID: 21530212 Free PMC article. Review.
Cited by
-
Immunogenicity evaluation of the HIV-1 Tat containing polyepitope DNA vaccine adjuvanted with CpG-ODNs in mice.Iran J Basic Med Sci. 2021 Mar;24(3):377-382. doi: 10.22038/ijbms.2021.52890.11923. Iran J Basic Med Sci. 2021. PMID: 33995949 Free PMC article.
-
Functional implications of the binding mode of a human conformation-dependent V2 monoclonal antibody against HIV.J Virol. 2014 Apr;88(8):4100-12. doi: 10.1128/JVI.03153-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478429 Free PMC article.
-
Enhanced Delivery and Potency of Self-Amplifying mRNA Vaccines by Electroporation in Situ.Vaccines (Basel). 2013 Aug 22;1(3):367-83. doi: 10.3390/vaccines1030367. Vaccines (Basel). 2013. PMID: 26344119 Free PMC article.
-
Elucidating the Kinetics of Expression and Immune Cell Infiltration Resulting from Plasmid Gene Delivery Enhanced by Surface Dermal Electroporation.Vaccines (Basel). 2013 Aug 28;1(3):384-97. doi: 10.3390/vaccines1030384. Vaccines (Basel). 2013. PMID: 26344120 Free PMC article.
-
Comparative analysis of enzymatically produced novel linear DNA constructs with plasmids for use as DNA vaccines.Gene Ther. 2014 Jul;21(7):645-52. doi: 10.1038/gt.2014.37. Epub 2014 May 15. Gene Ther. 2014. PMID: 24830436 Free PMC article.
References
-
- Arthos J, et al. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301–309 - PubMed
-
- Dolter KE, et al. 2011. Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation. Vaccine 29:795–803 - PubMed
-
- Fischer W, et al. 2007. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat. Med. 13:100–106 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

