Rescue of a genotype 4 human hepatitis E virus from cloned cDNA and characterization of intergenotypic chimeric viruses in cultured human liver cells and in pigs
- PMID: 22837416
- PMCID: PMC3541786
- DOI: 10.1099/vir.0.043711-0
Rescue of a genotype 4 human hepatitis E virus from cloned cDNA and characterization of intergenotypic chimeric viruses in cultured human liver cells and in pigs
Abstract
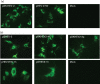
Hepatitis E virus (HEV) is an important but extremely understudied human pathogen. Genotypes 1 and 2 are restricted to humans, whereas genotypes 3 and 4 are zoonotic, infecting both humans and pigs. This report describes, for the first time, the successful rescue of infectious HEV in vitro and in vivo from cloned cDNA of a genotype 4 human HEV (strain TW6196E). The complete genomic sequence of the TW6196E virus was determined and a full-length cDNA clone (pHEV-4TW) was assembled. Capped RNA transcripts from the pHEV-4TW clone were replication competent in Huh7 cells and infectious in HepG2/C3A cells. Pigs inoculated intrahepatically with capped RNA transcripts from pHEV-4TW developed an active infection, as evidenced by faecal virus shedding and seroconversion, indicating the successful rescue of infectious genotype 4 HEV and cross-species infection of pigs by a genotype 4 human HEV. To demonstrate the utility of the genotype 4 HEV infectious clone and to evaluate the potential viral determinant(s) for species tropism, four intergenotypic chimeric clones were constructed by swapping various genomic regions between genotypes 1 and 4, and genotypes 1 and 3. All four chimeric clones were replication competent in Huh7 cells, but only the two chimeras with sequences swapped between genotypes 1 and 4 human HEVs produced viruses capable of infecting HepG2/C3A cells. None of the four chimeras was able to establish a robust infection in pigs. The availability of a genotype 4 HEV infectious clone affords an opportunity to delineate the molecular mechanisms of HEV cross-species infection in the future.
Figures


References
-
- Arankalle V. A., Chadha M. S., Tsarev S. A., Emerson S. U., Risbud A. R., Banerjee K., Purcell R. H. (1994). Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc Natl Acad Sci U S A 91, 3428–3432 10.1073/pnas.91.8.3428 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

