Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation
- PMID: 22837480
- PMCID: PMC3464911
- DOI: 10.1095/biolreprod.112.102673
Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation
Abstract
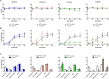
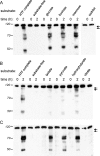
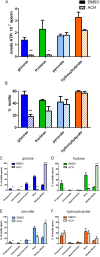
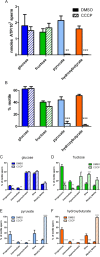
Although substantial evidence exists that sperm ATP production via glycolysis is required for mammalian sperm function and male fertility, conflicting reports involving multiple species have appeared regarding the ability of individual glycolytic or mitochondrial substrates to support the physiological changes that occur during capacitation. Several mouse models with defects in the signaling pathways required for capacitation exhibit reductions in sperm ATP levels, suggesting regulatory interactions between sperm metabolism and signal transduction cascades. To better understand these interactions, we conducted quantitative studies of mouse sperm throughout a 2-h in vitro capacitation period and compared the effects of single substrates assayed under identical conditions. Multiple glycolytic and nonglycolytic substrates maintained sperm ATP levels and comparable percentages of motility, but only glucose and mannose supported hyperactivation. These monosaccharides and fructose supported the full pattern of tyrosine phosphorylation, whereas nonglycolytic substrates supported at least partial tyrosine phosphorylation. Inhibition of glycolysis impaired motility in the presence of glucose, fructose, or pyruvate but not in the presence of hydroxybutyrate. Addition of an uncoupler of oxidative phosphorylation reduced motility with pyruvate or hydroxybutyrate as substrates but unexpectedly stimulated hyperactivation with fructose. Investigating differences between glucose and fructose in more detail, we demonstrated that hyperactivation results from the active metabolism of glucose. Differences between glucose and fructose appeared to be downstream of changes in intracellular pH, which rose to comparable levels during incubation with either substrate. Sperm redox pathways were differentially affected, with higher levels of associated metabolites and reactive oxygen species generated during incubations with fructose than during incubations with glucose.
Figures





Comment in
-
Sperm bioenergetics in a nutshell.Biol Reprod. 2012 Sep 28;87(3):72. doi: 10.1095/biolreprod.112.104109. Print 2012 Sep. Biol Reprod. 2012. PMID: 22914312 Free PMC article.
Similar articles
-
The role of glucose in supporting motility and capacitation in human spermatozoa.J Androl. 2001 Jul-Aug;22(4):680-95. J Androl. 2001. PMID: 11451366
-
Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa.Hum Reprod. 2011 Dec;26(12):3249-63. doi: 10.1093/humrep/der317. Epub 2011 Sep 23. Hum Reprod. 2011. PMID: 21946930 Free PMC article.
-
Glucose prevents the acquisition of the capacitated state in pig spermatozoa.Andrology. 2025 Mar;13(3):637-649. doi: 10.1111/andr.13691. Epub 2024 Jul 11. Andrology. 2025. PMID: 38993010 Free PMC article.
-
Energy metabolism and sperm function.Soc Reprod Fertil Suppl. 2007;65:309-25. Soc Reprod Fertil Suppl. 2007. PMID: 17644971 Review.
-
Protein phosphorylation in mammalian spermatozoa.Reproduction. 2003 Jan;125(1):17-26. doi: 10.1530/rep.0.1250017. Reproduction. 2003. PMID: 12622692 Review.
Cited by
-
Metabolomic and Transcriptomic Changes Underlying the Effects of L-Citrulline Supplementation on Ram Semen Quality.Animals (Basel). 2023 Jan 6;13(2):217. doi: 10.3390/ani13020217. Animals (Basel). 2023. PMID: 36670757 Free PMC article.
-
Amino Acids of Seminal Plasma Associated With Freezability of Bull Sperm.Front Cell Dev Biol. 2020 Jan 14;7:347. doi: 10.3389/fcell.2019.00347. eCollection 2019. Front Cell Dev Biol. 2020. PMID: 31993417 Free PMC article.
-
Structural analyses to identify selective inhibitors of glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme.Mol Hum Reprod. 2016 Jun;22(6):410-26. doi: 10.1093/molehr/gaw016. Epub 2016 Feb 26. Mol Hum Reprod. 2016. PMID: 26921398 Free PMC article.
-
Exploring the metabolome of seminal plasma in two different horse types: Light versus draft stallions.Reprod Domest Anim. 2023 Jan;58(1):109-116. doi: 10.1111/rda.14270. Epub 2022 Oct 3. Reprod Domest Anim. 2023. PMID: 36151924 Free PMC article.
-
Human Sperm Remain Motile After a Temporary Energy Restriction but do Not Undergo Capacitation-Related Events.Front Cell Dev Biol. 2021 Nov 12;9:777086. doi: 10.3389/fcell.2021.777086. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34869380 Free PMC article.
References
-
- Yanagimachi R. Mammalian fertilization. : Knobil E, Neill J. (eds.), The Physiology of Reproduction. New York: Raven Press; 1994: 189 317
-
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 2002; 53: 133 150 - PubMed
-
- Fraser LR. The “switching on” of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol Reprod Dev 2009; 77: 197 208 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

