How I treat plasma cell leukemia
- PMID: 22837533
- PMCID: PMC3757364
- DOI: 10.1182/blood-2012-05-408682
How I treat plasma cell leukemia
Abstract
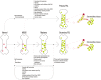
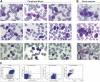
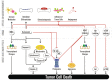
Primary plasma cell leukemia (pPCL) is a rare and aggressive plasma cell proliferative disorder with a very poor prognosis and with distinct biologic, clinical, and laboratory features. Compared with multiple myeloma, pPCL presents more often with extramedullary involvement, anemia, thrombocytopenia, hypercalcemia, elevated serum β(2)-microglobulin and lactate dehydrogenase levels, as well as impaired renal function. Many of the genetic aberrations observed in newly diagnosed pPCL are typically found in advanced multiple myeloma. These cytogenetic abnormalities and mutations lead to increased proliferation, enhanced inhibition of apoptosis, escape from immune surveillance, and independence from the BM microenvironment, with changes in expression of adhesion molecules or chemokine receptors. The outcome of pPCL has improved with the introduction of autologous stem cell transplantation and combination approaches with novel agents, including bortezomib and immunomodulatory drugs, such as lenalidomide. In this review, we provide an overview of currently available therapeutic options with recommendations of how these treatment modalities can best be used to improve outcome for plasma cell leukemia patients.
Figures


References
-
- Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia: report on 17 cases. Arch Intern Med. 1974;133(5):813–818. - PubMed
-
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. - PubMed
-
- Gluzinski A, Reichenstein M. Myeloma und leucaemia lymphatica plasmocellularis. Wien Klin Wochenschr. 1906;12:336–339.
-
- Avet-Loiseau H, Roussel M, Campion L, et al. Cytogenetic and therapeutic characterization of primary plasma cell leukemia: the IFM experience. Leukemia. 2012;26(1):158–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

