Modulation of Ligand Fluorescence by the Pt(II)/Pt(IV) Redox Couple
- PMID: 22837584
- PMCID: PMC3403686
- DOI: 10.1016/j.ica.2011.12.034
Modulation of Ligand Fluorescence by the Pt(II)/Pt(IV) Redox Couple
Abstract
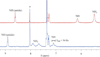
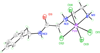
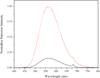
The dangling carboxylic acid moiety of the known platinum(II) complex, [Pt(edma)Cl(2)] (edma = ethylenediaminemonoacetic acid), was functionalized via amide coupling chemistry with benzyl amine and dansyl ethylenediamine to afford the derivatives [Pt(edBz)Cl(2)] (1) and [Pt(edDs)Cl(2)] (2). Subsequent oxidation of these platinum(II) complexes with iodobenzene dichloride in DMF yielded the respective platinum(IV) analogues, [Pt(edBz)Cl(4)] (3) and [Pt(edDs)Cl(4)] (4). All four platinum complexes were characterized by multinuclear NMR spectroscopy, IR spectroscopy, electrospray ionization mass spectrometry, and elemental analysis. In addition, compounds 1 and 3 were structurally characterized by X-ray crystallography. The photophysical properties of the compounds bearing the fluorescent dansyl moiety, 2 and 4, were evaluated. The emission quantum yields of 2 and 4 in DMF are 27% and 1.6%, respectively. This large difference in emission efficiency indicates that the platinum(IV) center in 4 is more effective at quenching the dansyl-based fluorescence than the platinum(II) center in 2. Time-dependent density functional theory calculations indicate that 4 has several low-lying singlet excited states that energetically lie below the primary radiation-accessible excited state of the dansyl fluorophore. These low-energy excited states may offer non-radiative decay pathways that lower the overall emission quantum yield. Treatment of 4 with biologically relevant reducing agents in pH 7.4 phosphate-buffered saline induces a 6.3-fold increase in emission intensity. These results demonstrate that 4 and future derivatives thereof may be useful for imaging the reduction of platinum(IV) complexes in living systems, chemistry of importance for future platinum-based anticancer drug strategies.
Figures
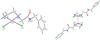




Similar articles
-
Importance of platinum(II)-assisted platinum(IV) substitution for the oxidation of guanosine derivatives by platinum(IV) complexes.Inorg Chem. 2008 Feb 18;47(4):1352-60. doi: 10.1021/ic701868b. Epub 2008 Jan 26. Inorg Chem. 2008. PMID: 18220340
-
Oxidation of the Platinum(II) Anticancer Agent [Pt{(p-BrC6F4)NCH2CH2NEt2}Cl(py)] to Platinum(IV) Complexes by Hydrogen Peroxide.Molecules. 2023 Sep 1;28(17):6402. doi: 10.3390/molecules28176402. Molecules. 2023. PMID: 37687231 Free PMC article.
-
Synthesis, characterization, and photophysical properties of three platinum(II) complexes bearing fluorescent analogues of the Di-2-pyridylmethane ligand.Inorg Chem. 2010 Jun 7;49(11):5303-15. doi: 10.1021/ic100411p. Inorg Chem. 2010. PMID: 20423108 Free PMC article.
-
Luminescent charge-transfer platinum(II) metallacycle.Inorg Chem. 2007 Oct 15;46(21):8771-83. doi: 10.1021/ic701103u. Epub 2007 Sep 15. Inorg Chem. 2007. PMID: 17867679
-
Spectroscopic and luminescence studies on square-planar platinum(II) complexes with anionic tridentate 3-bis(2-pyridylimino)isoindoline derivatives.Inorg Chem. 2010 Mar 1;49(5):2210-21. doi: 10.1021/ic902019s. Inorg Chem. 2010. PMID: 20104854
Cited by
-
NanoSIMS combined with fluorescence microscopy as a tool for subcellular imaging of isotopically labeled platinum-based anticancer drugs.Chem Sci. 2014 Jun 6;5(8):3135-3143. doi: 10.1039/c3sc53426j. eCollection 2014 Jun 30. Chem Sci. 2014. PMID: 35919909 Free PMC article.
-
Pt(IV) prodrugs designed to bind non-covalently to human serum albumin for drug delivery.J Am Chem Soc. 2014 Jun 18;136(24):8790-8. doi: 10.1021/ja5038269. Epub 2014 Jun 6. J Am Chem Soc. 2014. PMID: 24902769 Free PMC article.
-
Nanoscale drug delivery platforms overcome platinum-based resistance in cancer cells due to abnormal membrane protein trafficking.ACS Nano. 2013 Dec 23;7(12):10452-64. doi: 10.1021/nn405004f. Epub 2013 Dec 10. ACS Nano. 2013. PMID: 24219825 Free PMC article. Review.
-
Cell-permeable lanthanide-platinum(IV) anti-cancer prodrugs.Dalton Trans. 2021 Jun 29;50(25):8761-8767. doi: 10.1039/d1dt01688a. Dalton Trans. 2021. PMID: 34080595 Free PMC article.
-
Oxidative halogenation of cisplatin and carboplatin: synthesis, spectroscopy, and crystal and molecular structures of Pt(IV) prodrugs.Dalton Trans. 2015 Jan 7;44(1):119-29. doi: 10.1039/c4dt02627f. Dalton Trans. 2015. PMID: 25367395 Free PMC article.
References
-
- Jung Y, Lippard SJ. Chem. Rev. 2007;107:1387–1407. - PubMed
-
- Kelland L. Nat. Rev. Cancer. 2007;7:573–584. - PubMed
-
- Harper BW, Krause-Heuer AM, Grant MP, Manohar M, Garbutcheon-Singh KB, Aldrich-Wright JR. Chem. Eur. J. 2010;16:7064–7077. - PubMed
-
- Hall MD, Hambley TW. Coord. Chem. Rev. 2002;232:49–67.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
