Therapeutic angiogenesis: controlled delivery of angiogenic factors
- PMID: 22838066
- PMCID: PMC3564557
- DOI: 10.4155/tde.12.50
Therapeutic angiogenesis: controlled delivery of angiogenic factors
Abstract
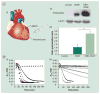
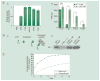
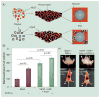
Therapeutic angiogenesis aims at treating ischemic diseases by generating new blood vessels from existing vasculature. It relies on delivery of exogenous factors to stimulate neovasculature formation. Current strategies using genes, proteins and cells have demonstrated efficacy in animal models. However, clinical translation of any of the three approaches has proved to be challenging for various reasons. Administration of angiogenic factors is generally considered safe, according to accumulated trials, and offers off-the-shelf availability. However, many hurdles must be overcome before therapeutic angiogenesis can become a true human therapy. This article will highlight protein-based therapeutic angiogenesis, concisely review recent progress and examine critical challenges. We will discuss growth factors that have been widely utilized in promoting angiogenesis and compare their targets and functions. Lastly, since bolus injection of free proteins usually result in poor outcomes, we will focus on controlled release of proteins.
Figures




References
-
- Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. - PubMed
-
- Hedman M, Hartikainen J, Yla-Herttuala S. Progress and prospects: hurdles to cardiovascular gene therapy clinical trials. Gene Ther. 2011;18(8):743–749. Summarizes recent progress and future direction of angiogenic gene therapy. - PubMed
-
- Ishikawa K, Tilemann L, Fish K, Hajjar RJ. Gene delivery methods in cardiac gene therapy. J Gene Med. 2011;13(10):566–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
