Hydroxy decenoic acid down regulates gtfB and gtfC expression and prevents Streptococcus mutans adherence to the cell surfaces
- PMID: 22839724
- PMCID: PMC3495742
- DOI: 10.1186/1476-0711-11-21
Hydroxy decenoic acid down regulates gtfB and gtfC expression and prevents Streptococcus mutans adherence to the cell surfaces
Abstract
Background: 10-Hydroxy-2-decenoic acid, an unsaturated fatty acid is the most active and unique component to the royal jelly that has antimicrobial properties. Streptococcus mutans is associated with pathogenesis of oral cavity, gingivoperiodontal diseases and bacteremia following dental manipulations. In the oral cavity, S. mutans colonize the soft tissues including tongue, palate, and buccal mucosa. When considering the role of supragingival dental plaque in caries, the proportion of acid producing bacteria (particularly S. mutans), has direct relevance to the pathogenicity of the plaque. The genes that encode glucosyltransferases (gtfs) especially gtfB and gtfC are important in S. mutans colonization and pathogenesis. This study investigated the hydroxy-decenoic acid (HDA) effects on gtfB and gtfC expression and S. mutans adherence to cells surfaces.
Methods: Streptococcus mutans was treated by different concentrations of HPLC purified HDA supplied by Iran Beekeeping and Veterinary Association. Real time RT-PCR and western blot assays were conducted to evaluate gtfB and gtfC genes transcription and translation before and after HDA treatment. The bacterial attachment to the cell surfaces was evaluated microscopically.
Results: 500 μg ml-1 of HDA inhibited gtfB and gtfC mRNA transcription and its expression. The same concentration of HDA decreased 60% the adherence of S. mutans to the surface of P19 cells.
Conclusion: Hydroxy-decenoic acid prevents gtfB and gtfC expression efficiently in the bactericide sub-concentrations and it could effectively reduce S. mutans adherence to the cell surfaces. In the future, therapeutic approaches to affecting S. mutans could be selective and it's not necessary to put down the oral flora completely.
Figures
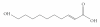




References
-
- Hamilton IR, Bowden GH. In: Encyclopedia of microbiology. Lederberg J, editor. San Diego, Calif: Academic Press; 2000. Oral microbiology; pp. 466–480.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

