Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria)
- PMID: 22839781
- PMCID: PMC3473239
- DOI: 10.1186/1741-7007-10-65
Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria)
Abstract
Background: The morphological peculiarities of turtles have, for a long time, impeded their accurate placement in the phylogeny of amniotes. Molecular data used to address this major evolutionary question have so far been limited to a handful of markers and/or taxa. These studies have supported conflicting topologies, positioning turtles as either the sister group to all other reptiles, to lepidosaurs (tuatara, lizards and snakes), to archosaurs (birds and crocodiles), or to crocodilians. Genome-scale data have been shown to be useful in resolving other debated phylogenies, but no such adequate dataset is yet available for amniotes.
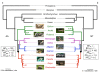
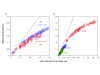
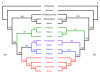
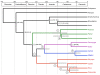
Results: In this study, we used next-generation sequencing to obtain seven new transcriptomes from the blood, liver, or jaws of four turtles, a caiman, a lizard, and a lungfish. We used a phylogenomic dataset based on 248 nuclear genes (187,026 nucleotide sites) for 16 vertebrate taxa to resolve the origins of turtles. Maximum likelihood and Bayesian concatenation analyses and species tree approaches performed under the most realistic models of the nucleotide and amino acid substitution processes unambiguously support turtles as a sister group to birds and crocodiles. The use of more simplistic models of nucleotide substitution for both concatenation and species tree reconstruction methods leads to the artefactual grouping of turtles and crocodiles, most likely because of substitution saturation at third codon positions. Relaxed molecular clock methods estimate the divergence between turtles and archosaurs around 255 million years ago. The most recent common ancestor of living turtles, corresponding to the split between Pleurodira and Cryptodira, is estimated to have occurred around 157 million years ago, in the Upper Jurassic period. This is a more recent estimate than previously reported, and questions the interpretation of controversial Lower Jurassic fossils as being part of the extant turtles radiation.
Conclusions: These results provide a phylogenetic framework and timescale with which to interpret the evolution of the peculiar morphological, developmental, and molecular features of turtles within the amniotes.
Figures
References
-
- Rieppel O. In: Biology of turtles. Wyneken J, Godfrey MH, Bels V, editor. Boca Raton, FL: CRC Press, Taylor and & Francis Group; 2007. The relationships of turtles within amniotes; pp. 345–353.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

